Biology:Human parainfluenza viruses
| Human parainfluenza viruses | |
|---|---|
| |
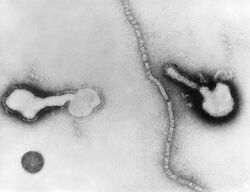
| Transmission electron micrograph of a parainfluenza virus. Two intact particles and free filamentous nucleocapsid | |
| Scientific classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Paramyxoviridae |
| Groups included | |
| |
| Cladistically included but traditionally excluded taxa | |
| |
Human parainfluenza viruses (HPIVs) are the viruses that cause human parainfluenza. HPIVs are a paraphyletic group of four distinct single-stranded RNA viruses belonging to the Paramyxoviridae family. These viruses are closely associated with both human and veterinary disease.[2] Virions are approximately 150–250 nm in size and contain negative sense RNA with a genome encompassing about 15,000 nucleotides.[3]
The viruses can be detected via cell culture, immunofluorescent microscopy, and PCR.[4] HPIVs remain the second main cause of hospitalisation in children under 5 years of age for a respiratory illness (only Respiratory syncytial virus (RSV) causes more respiratory hospitalisations for this age group).[5]
Classification
The first HPIV was discovered in the late 1950s. The taxonomic division is broadly based on antigenic and genetic characteristics, forming four major serotypes or clades, which today are considered distinct viruses.[6] These include:
| Virus | GenBank acronym | NCBI taxonomy | Notes |
|---|---|---|---|
| Human parainfluenza virus type 1 | HPIV-1 | 12730 | Most common cause of croup |
| Human parainfluenza virus type 2 | HPIV-2 | 11212 | Causes croup and other upper and lower respiratory tract illnesses |
| Human parainfluenza virus type 3 | HPIV-3 | 11216 | Associated with bronchiolitis and pneumonia |
| Human parainfluenza virus type 4 | HPIV-4 | 11203 | Includes subtypes 4a and 4b |
HPIVs belong to two genera: Respirovirus (HPIV-1 & HPIV-3) and Rubulavirus (HPIV-2 & HPIV-4).[3]
Viral structure and organisation
HPIVs are characterised by producing enveloped virions and containing single stranded negative sense RNA.[3] Non-infectious virions have also been reported to contain RNA with positive polarity.[3] HPIV genomes are about 15,000 nucleotides in length and encode six key structural proteins.[3]
The structural gene sequence of HPIVs is as follows: 3′-NP-P-M-F-HN-L-5′ (the protein prefixes and further details are outlined in the table below).[7]
| Structural protein | Location | Function |
|---|---|---|
| Hemagglutinin-neuraminidase (HN) | Envelope | Attachment and cell entry |
| Fusion Protein (F) | Envelope | Fusion and cell entry |
| Matrix Protein (M) | Within the envelope | Assembly |
| Nucleoprotein (NP) | Nucleocapsid | Forms a complex with the RNA genome |
| Phosphoprotein (P) | Nucleocapsid | Forms as part of RNA polymerase complex |
| Large Protein (L) | Nucleocapsid | Forms as part of RNA polymerase complex |
With the advent of reverse genetics, it has been found that the most efficient human parainfluenza viruses (in terms of replication and transcription) have a genome nucleotide total that is divisible by the number 6. This has led to the "rule of six" being coined. Exceptions to this rule have been found and its exact advantages are not fully understood.[8]
Electrophoresis has shown that the molecular weight (MW) of the proteins for the four HPIVs are similar (with the exception of the phosphoprotein, which shows significant variation).[3][9]
Viral entry and replication
Viral replication is initiated only after successful entry into a cell by attachment and fusion between the virus and the host cell lipid membrane. Viral RNA (vRNA) is initially associated with nucleoprotein (NP), phosphoprotein (P) and the large protein (L). The hemagglutinin–neuraminidase (HN) is involved with viral attachment and thus hemadsorption and hemagglutination. Furthermore, the fusion (F) protein is important in aiding the fusion of the host and viral cellular membranes, eventually forming syncytia.[10]
Initially the F protein is in an inactive form (F0) but can be cleaved by proteolysis to form its active form, F1 and F2, linked by di-sulphide bonds. Once complete, this is followed by the HPIV nucleocapsid entering the cytoplasm of the cell. Subsequently, genomic transcription occurs using the viruses own 'viral RNA-dependent RNA polymerase' (L protein). The cell's own ribosomes are then tasked with translation, forming the viral proteins from the viral mRNA.[10]
Towards the end of the process, (after the formation of the viral proteins) the replication of the viral genome occurs. Initially, this occurs with the formation of a positive-sense RNA (intermediate step, necessary for producing progeny), and finally, negative-sense RNA is formed which is then associated with the nucleoprotein. This may then be either packaged and released from the cell by budding or used for subsequent rounds of transcription and replication.[11]
The observable and morphological changes that can be seen in infected cells include the enlargement of the cytoplasm, decreased mitotic activity and 'focal rounding', with the potential formation of multi-nucleate cells (syncytia).[12]
The pathogenicity of HPIVs is mutually dependent on the viruses having the correct accessory proteins that are able to elicit anti-interferon properties. This is a major factor in the clinical significance of disease.[11]
Host range
The main host remains the human. However, infections have been induced in other animals (both under natural and experimental situations), although these were always asymptomatic.[13]
Clinical significance
It is estimated that there are 5 million children with lower respiratory infections (LRI) each year in the United States alone.[14] HPIV-1, HPIV-2 and HPIV-3 have been linked with up to a third of these infections.[15] Upper respiratory infections (URI) are also important in the context of HPIV, however, they are caused to a lesser extent by the virus.[16] The highest rates of serious HPIV illnesses occur among young children, and surveys have shown that about 75% of children aged 5 or older have antibodies to HPIV-1.[citation needed]
For infants and young children, it has been estimated that about 25% will develop "clinically significant disease".[17]
Repeated infection throughout the life of the host is not uncommon and symptoms of later breakouts include upper respiratory tract illness, such as cold and a sore throat.[3] The incubation period for all four serotypes is 1 to 7 days.[18] In immunosuppressed people, parainfluenza virus infections can cause severe pneumonia, which can be fatal.[19]
HPIV-1 and HPIV-2 have been demonstrated to be the principal causative agent behind croup (laryngotracheobronchitis), which is a viral disease of the upper airway and is mainly problematic in children aged 6–48 months of age.[20][21] Biennial epidemics starting in Autumn are associated with both HPIV-1 and 2; however, HPIV-2 can also have yearly outbreaks.[14] Additionally, HPIV-1 tends to cause biennial outbreaks of croup in the Fall. In the United States, large peaks have presently been occurring during odd-numbered years.[citation needed]
HPIV-3 has been closely associated with bronchiolitis and pneumonia and principally targets those aged <1 year.[22]
HPIV-4 remains infrequently detected. However, it is now believed to be more common than previously thought, but is less likely to cause severe disease. By the age of 10, the majority of children are seropositive for HPIV-4 infection which may be indicative of a large proportion of asymptomatic or mild infections.[3]
Important epidemiological factors that are associated with a higher risk of infection and mortality are those who are immuno-compromised and may be taken ill with more extreme forms of LRI.[13] Associations between HPIVs and neurologic disease are known; for example, hospitalisation with certain HPIVs has a strong association with febrile seizures.[23] HPIV-4B has the strongest association (up to 62%) followed by HPIV-3 and -1.[3]
HPIVs have also been linked with rare cases of viral meningitis[24] and Guillain–Barré syndrome.[12]
HPIVs are spread from person to person ('horizontal transmission') by contact with infected secretions through respiratory droplets or contaminated surfaces or objects. Infection can occur when infectious material contacts mucous membranes of the eyes, mouth, or nose, and possibly through the inhalation of droplets generated by a sneeze or cough. HPIVs can remain infectious in airborne droplets for over an hour.[citation needed]
Overall, HPIVs remain best known for its effects on the respiratory system and this appears to be where the majority of the focus has been upon.[citation needed]
Airway inflammation
The inflammation of the airway is a common attribute of HPIV infection. It is believed to occur due to the large scale up-regulation of inflammatory cytokines. Common cytokines, expected to be up-regulated, include IFN–α, various other interleukins (IL–2, IL-6) and TNF–α. Various chemokines and inflammatory proteins are also believed to be associated with the common symptoms of HPIV infection.[12]
Recent evidence seems to suggest that virus-specific antibodies (IgE) may be responsible for mediating large-scale releases of histamine in the trachea, which are believed to cause croup.[12][25]
Immunology
The body's primary defense against HPIV infection is adaptive immunity involving both humoral and cellular immunity. With humoral immunity, antibodies that bind to the surface viral proteins HN and F protect against later infection.[26] Patients with defective cell-mediated immunity also experience more severe infection, suggesting that T cells are important in clearing infection.[12]
Diagnosis
Diagnosis can be made in several ways, encompassing a range of multi-faceted techniques:[4]
- Isolation and detection of the virus in cell culture.
- Detection of viral antigens directly within bodily respiratory tract secretions using immunofluorescence, enzyme immunoassays or fluoroimmunoassays.
- Polymerase chain reaction (PCR).
- Analysis of specific IgG antibodies showing a subsequent rise in titre following infection (using paired serum specimens).
Because of the similarity in terms of the antigenic profile between the viruses, hemagglutination assay (HA) or hemadsorption inhibition (HAdI) processes are often used. Both complement fixation, neutralisation, and enzyme linked immunosorbent assays – ELISA, can also be used to aid in the process of distinguishing between viral serotypes.[3]
Morbidity and mortality
Mortality caused by HPIVs in developed regions of the world remains rare. Where mortality has occurred, it is principally in the three core risk groups (very young, elderly and immuno-compromised). Long-term changes can however be associated with airway remodeling and are believed to be a significant cause of morbidity.[27] The exact associations between HPIVs and diseases such as chronic obstructive pulmonary disease (COPD) are still being investigated.[28]
In developing regions of the world, preschool children remain the highest mortality risk group. Mortality may be a consequence of primary viral infection or secondary problems, such as bacterial infection. Predispositions, such as malnutrition and other deficiencies, may further elevate the chances of mortality associated with infection.[12]
Overall, LRIs cause approximately 25–30% of total deaths in preschool children in the developing world. HPIVs are believed to be associated with 10% of all LRI cases, thus remaining a significant cause of mortality.[12]
Risk factors
Numerous factors have been suggested and linked to a higher risk of acquiring the infection, inclusive of malnutrition, vitamin A deficiency, absence of breastfeeding during the early stages of life, environmental pollution and overcrowding.[29]
Prevention
Despite decades of research, no vaccines currently exist.[30]
Recombinant technology has however been used to target the formation of vaccines for HPIV-1, -2 and -3 and has taken the form of several live-attenuated intranasal vaccines. Two vaccines in particular were found to be immunogenic and well tolerated against HPIV-3 in phase I trials. HPIV-1 and -2 vaccine candidates remain less advanced.[17]
Vaccine techniques which have been used against HPIVs are not limited to intranasal forms, but also viruses attenuated by cold passage, host range attenuation, chimeric construct vaccines and also introducing mutations with the help of reverse genetics to achieve attenuation.[31]
Maternal antibodies may offer some degree of protection against HPIVs during the early stages of life via the colostrum in breast milk.[32]
Medication
Ribavirin is one medication which has shown good potential for the treatment of HPIV-3 given recent in-vitro tests (in-vivo tests show mixed results).[12] Ribavirin is a broad-spectrum antiviral, and as of 2012, was being administered to those who are severely immuno-compromised, despite the lack of conclusive evidence for its benefit.[12] Protein inhibitors and novel forms of medication have also been proposed to relieve the symptoms of infection.[13]
Furthermore, antibiotics may be used if a secondary bacterial infection develops. Corticosteroid treatment and nebulizers are also a first line choice against croup if breathing difficulties ensue.[12]
Interactions with the environment
Parainfluenza viruses last only a few hours in the environment and are inactivated by soap and water. Furthermore, the virus can also be easily destroyed using common hygiene techniques and detergents, disinfectants and antiseptics.[4]
Environmental factors which are important for HPIV survival are pH, humidity, temperature and the medium within which the virus is found. The optimal pH is around the physiologic pH values (7.4 to 8.0), whilst at high temperatures (above 37 °C) and low humidity, infectivity reduces.[33]
The majority of transmission has been linked to close contact, especially in nosocomial infections. Chronic care facilities and doctors' surgeries are also known to be transmission 'hotspots' with transmission occurring via aerosols, large droplets and also fomites (contaminated surfaces).[34]
The exact infectious dose remains unknown.[13]
Economic burden
In economically disadvantaged regions of the world, HPIV infection can be measured in terms of mortality. In the developed world where mortality remains rare, the economic costs of the infection can be estimated. Estimates from the US are suggestive of a cost (based on extrapolation) in the region of $200 million per annum.[3]
References
- ↑ "Virus Taxonomy: 2018 Release" (in en). October 2018. https://ictv.global/taxonomy.
- ↑ "Biology of parainfluenza viruses". Clin. Microbiol. Rev. 7 (2): 265–275. April 1994. doi:10.1128/CMR.7.2.265. PMID 8055470.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 Henrickson, KJ (April 2003). "Parainfluenza viruses". Clinical Microbiology Reviews 16 (2): 242–264. doi:10.1128/CMR.16.2.242-264.2003. PMID 12692097.
- ↑ 4.0 4.1 4.2 "Human Parainfluenza Viruses". Centers for Disease Control and Prevention (2011). https://www.cdc.gov/ncidod/dvrd/revb/respiratory/hpivfeat.htm.
- ↑ Schmidt, Alexander; Anne Schaap-Nutt; Emmalene J Bartlett; Henrick Schomacker; Jim Boonyaratanakornkit; Ruth A Karron; Peter L Collins (1 February 2011). "Progress in the development of human parainfluenza virus vaccines". Expert Review of Respiratory Medicine 5 (4): 515–526. doi:10.1586/ers.11.32. PMID 21859271.
- ↑ Baron, S.; Enders, G. (1996). "Paramyxoviruses". Parainfluenza Viruses. University of Texas Medical Branch at Galveston. ISBN 9780963117212. https://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=mmed.section.3128. Retrieved 2009-03-15.
- ↑ Hunt, Dr. Margaret. "PARAINFLUENZA, RESPIRATORY SYNCYTIAL AND ADENO VIRUSES". Reference.MD. http://pathmicro.med.sc.edu/virol/para-rsv-aden-ver2.htm.
- ↑ Vulliémoz, D; Roux, L (May 2001). "'Rule of six': how does the Sendai virus RNA polymerase keep count?". Journal of Virology 75 (10): 4506–4518. doi:10.1128/JVI.75.10.4506-4518.2001. PMID 11312321.
- ↑ Henrickson, K. J (2003). "Parainfluenza Viruses". Clinical Microbiology Reviews 16 (2): 242–264. doi:10.1128/CMR.16.2.242-264.2003. PMID 12692097.
- ↑ 10.0 10.1 Moscona, A (July 2005). "Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease". The Journal of Clinical Investigation 115 (7): 1688–1698. doi:10.1172/JCI25669. PMID 16007245.
- ↑ 11.0 11.1 "Parainfluenza Viruses". Encyclopedia of Life Sciences. Wiley. 2011. doi:10.1002/9780470015902.a0001078.pub3. ISBN 978-0470016176.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 "Parainfluenza Virus: Epidemiology". eMedicine. http://emedicine.medscape.com/article/224708-overview#a0199.
- ↑ 13.0 13.1 13.2 13.3 "HUMAN PARAINFLUENZA VIRUS". Public Health Agency of Canada. 2011-04-19. http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/parainfluenza-eng.php.
- ↑ 14.0 14.1 Henrickson, KJ; Kuhn, SM; Savatski, LL (May 1994). "Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children". Clinical Infectious Diseases 18 (5): 770–9. doi:10.1093/clinids/18.5.770. PMID 8075269.
- ↑ Denny, FW; Clyde WA, Jr (May 1986). "Acute lower respiratory tract infections in nonhospitalized children". The Journal of Pediatrics 108 (5 Pt 1): 635–46. doi:10.1016/S0022-3476(86)81034-4. PMID 3009769.
- ↑ "Acute Respiratory Infections". WHO. https://www.who.int/vaccine_research/diseases/ari/en/index2.html.
- ↑ 17.0 17.1 Durbin, AP; Karron, RA (December 15, 2003). "Progress in the development of respiratory syncytial virus and parainfluenza virus vaccines". Clinical Infectious Diseases 37 (12): 1668–1677. doi:10.1086/379775. PMID 14689350.
- ↑ "General information: human parainfluenza viruses". Health Protection Agency. 27 August 2008. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/HumanParainfluenzaViruses/GeneralInformation/.
- ↑ "Orthomyxoviral and paramyxoviral infections in transplant patients". Infect. Dis. Clin. North Am. 9 (4): 987–1003. December 1995. doi:10.1016/S0891-5520(20)30712-1. PMID 8747776.
- ↑ "CDC - Human Parainfluenza Viruses: Common cold and croup". https://www.cdc.gov/ncidod/dvrd/revb/respiratory/hpivfeat.htm.
- ↑ "Croup Background". http://emedicine.medscape.com/article/962972-overview.
- ↑ "Parainfluenza Virus Review". http://misc.medscape.com/pi/android/medscapeapp/html/A224708-business.html.
- ↑ "Parainfluenza Viruses—New Epidemiology and Vaccine Developments". Touch Infectious Disease. http://www.touchinfectiousdisease.com/articles/parainfluenza-viruses-new-epidemiology-and-vaccine-developments-0?page=0,2.
- ↑ Arguedas, A; Stutman, HR; Blanding, JG (March 1990). "Parainfluenza type 3 meningitis. Report of two cases and review of the literature". Clinical Pediatrics 29 (3): 175–178. doi:10.1177/000992289002900307. PMID 2155085.
- ↑ "Human Parainfluenza Viruses (HPIV) and Other Parainfluenza Viruses: Background, Pathophysiology, Etiology". 17 October 2021. https://emedicine.medscape.com/article/224708-overview#showall.
- ↑ Branche, Angela; Falsey, Ann (2016-08-03). "Parainfluenza Virus Infection" (in en). Seminars in Respiratory and Critical Care Medicine 37 (4): 538–554. doi:10.1055/s-0036-1584798. ISSN 1069-3424. PMID 27486735.
- ↑ Dimopoulos, G; Lerikou, M; Tsiodras, S; Chranioti, A; Perros, E; Anagnostopoulou, U; Armaganidis, A; Karakitsos, P (February 2012). "Viral epidemiology of acute exacerbations of chronic obstructive pulmonary disease". Pulmonary Pharmacology & Therapeutics 25 (1): 12–8. doi:10.1016/j.pupt.2011.08.004. PMID 21983132.
- ↑ Beckham, JD; Cadena, A; Lin, J; Piedra, PA; Glezen, WP; Greenberg, SB; Atmar, RL (May 2005). "Respiratory viral infections in patients with chronic, obstructive pulmonary disease". The Journal of Infection 50 (4): 322–30. doi:10.1016/j.jinf.2004.07.011. PMID 15845430.
- ↑ Berman, S (May–Jun 1991). "Epidemiology of acute respiratory infections in children of developing countries". Reviews of Infectious Diseases 13 (Suppl 6): S454–62. doi:10.1093/clinids/13.supplement_6.s454. PMID 1862276.
- ↑ "Current status of vaccines for parainfluenza virus infections". Pediatr. Infect. Dis. J. 27 (10 Suppl): S123–5. October 2008. doi:10.1097/INF.0b013e318168b76f. PMID 18820572.
- ↑ "Parainfluenza Viruses". eLS. http://www.els.net/WileyCDA/ElsArticle/refId-a0001078.html.
- ↑ "Definition of Human parainfluenza virus". MedicineNet. http://www.medterms.com/script/main/art.asp?articlekey=31631.
- ↑ HAMBLING, MH (December 1964). "Survival of the Respiratory Syncytial Virus During Storage Under Various Conditions". British Journal of Experimental Pathology 45 (6): 647–55. PMID 14245166.
- ↑ "Common Cold, Croup and Human Parainfluenza Viruses: Symptoms and Prevention.". NewsFlu. http://www.newsflu.com/tag/cold/.
Further reading
- "Parainfluenza viruses". Clin. Microbiol. Rev. 16 (2): 242–64. 2003. doi:10.1128/cmr.16.2.242-264.2003. PMID 12692097.
- Human Parainfluenza Viruses (HPIVs)
External links
- Human Parainfluenza Viruses – information provided by the CDC
| Classification | |
|---|---|
| External resources |
 |