Chemistry:Potassium bicarbonate
| |
| Names | |
|---|---|
| IUPAC name
potassium hydrogencarbonate
| |
| Other names
potassium hydrogencarbonate, potassium acid carbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| 4535309 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| KHCO3 | |
| Molar mass | 100.115 g/mol |
| Appearance | white crystals |
| Odor | odorless |
| Density | 2.17 g/cm3 |
| Melting point | 292 °C (558 °F; 565 K) (decomposes) |
| 22.4 g/100 mL (20 °C)[1] | |
| Solubility | practically insoluble in alcohol |
| Acidity (pKa) | 10.329[2]
6.351 (carbonic acid)[2] |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-963.2 kJ/mol |
| Pharmacology | |
| 1=ATC code }} | A12BA04 (WHO) |
| Hazards[3] | |
| Safety data sheet | MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P280, P302+352, P304+340, P305+351+338, P312, P332+313, P362, P403+233, P405 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-Flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
> 2000 mg/kg (rat, oral) |
| Related compounds | |
Other anions
|
Potassium carbonate |
Other cations
|
Sodium bicarbonate Ammonium bicarbonate |
Related compounds
|
Potassium bisulfate Monopotassium phosphate Dipotassium phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
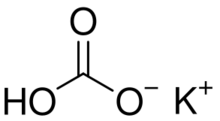
Potassium bicarbonate (IUPAC name: potassium hydrogencarbonate, also known as potassium acid carbonate) is the inorganic compound with the chemical formula KHCO3. It is a white solid.[1]
Production and reactivity
It is manufactured by treating an aqueous solution of potassium carbonate with carbon dioxide:[1]
- K2CO3 + CO2 + H2O → 2 KHCO3
Decomposition of the bicarbonate occurs between 100 and 120 °C (212 and 248 °F):
- 2 KHCO3 → K2CO3 + CO2 + H2O
This reaction is employed to prepare high purity potassium carbonate.
Uses
Food and drink
This compound is a source of carbon dioxide for leavening in baking. It can substitute for baking soda (sodium bicarbonate) for those with a low-sodium diet,[4] and it is an ingredient in low-sodium baking powders.[5][6]
As an inexpensive, nontoxic base, it is widely used in diverse application to regulate pH or as a reagent. Examples include as buffering agent in medications, an additive in winemaking.
Potassium bicarbonate is often added to bottled water to improve taste,[7] and is also used in club soda.
Medical uses and health
Higher potassium intake may prevent development of kidney stone disease.[8] Higher potassium intake is associated with a reduced risk of stroke.[9]
Fire extinguishers
Potassium bicarbonate is used as a fire suppression agent ("BC dry chemical") in some dry chemical fire extinguishers, as the principal component of the Purple-K dry chemical, and in some applications of condensed aerosol fire suppression. It is the only dry chemical fire suppression agent recognized by the U.S. National Fire Protection Association for firefighting at airport crash rescue sites. It is about twice as effective in fire suppression as sodium bicarbonate.[10]
Agriculture
Potassium bicarbonate has widespread use in crops, especially for neutralizing acidic soil.[11]
Potassium bicarbonate is an effective fungicide against powdery mildew and apple scab, allowed for use in organic farming.[12][13][14][15] Potassium bicarbonate is a contact killer for Spanish moss when mixed 1/4 cup per gallon.[16]
History
The word saleratus, from Latin sal æratus meaning "aerated salt", first used in the nineteenth century, refers to both potassium bicarbonate and sodium bicarbonate.[17]
References
- ↑ 1.0 1.1 1.2 H. Schultz; G. Bauer; E. Schachl; F. Hagedorn; P. Schmittinger (2005). "Potassium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_039. ISBN 3-527-30673-0.
- ↑ 2.0 2.1 Goldberg, Robert N.; Kishore, Nand; Lennen, Rebecca M. (2003). "Thermodynamic quantities for the ionization reactions of buffers in water". in David R. Lide. CRC handbook of chemistry and physics (84th ed.). Boca Raton, FL: CRC Press. pp. 7–13. ISBN 978-0-8493-0595-5. https://books.google.com/books?id=q2qJId5TKOkC&pg=PP9. Retrieved 6 March 2011.
- ↑ "Potassium bicarbonate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/516893#section=Safety-and-Hazards.
- ↑ "Potassium Bicarbonate". Cengage. https://www.encyclopedia.com/science/academic-and-educational-journals/potassium-bicarbonate.
- ↑ "Home cooking with less salt". Harvard University. March 2020. https://www.health.harvard.edu/heart-health/home-cooking-with-less-salt.
- ↑ Wilkens, Katy G. (15 December 2018). "You Have the (Baking) Power with Low-Sodium Baking Powders". Aging & Disability Services for Seattle & King County. https://www.agingkingcounty.org/2018/12/14/you-have-the-baking-power-with-low-sodium-baking-powders/.
- ↑ "Why Your Bottled Water Contains Four Different Ingredients". Time Magazine. July 24, 2014. http://time.com/3029191/bottled-water-ingredients-nutrition-health/.
- ↑ "Beneficial effects of potassium on human health". Physiologia Plantarum 133 (4): 725–735. 2008. doi:10.1111/j.1399-3054.2007.01033.x. PMID 18724413.
- ↑ "Meta-Analysis of Potassium Intake and the Risk of Stroke". Journal of the American Heart Association 5 (10): e004210. 2016. doi:10.1161/JAHA.116.004210. PMID 27792643.
- ↑ "Purple-K-Powder". US Naval Research Laboratory. http://www.nrl.navy.mil/content.php?P=PURPLEK.
- ↑ "Potassium Bicarbonate Handbook". Armand Products Company. http://www.armandproducts.com/pdfs/potbivs6.pdf.
- ↑ "Use of Baking Soda as a Fungicide". http://attra.ncat.org/attra-pub/bakingsoda.html.
- ↑ "Powdery Mildew - Sustainable Gardening Australia". http://www.sgaonline.org.au/info_powderymildew.html.
- ↑ "Organic Fruit Production in Michigan". http://www.msue.msu.edu/objects/content_revision/download.cfm/item_id.163744/workspace_id.112004/Organic%20Fruit%20Production%20Fruit%20Production.doc.
- ↑ "Efficacy of Armicarb (potassium bicarbonate) against scab and sooty blotch on apples". http://orgprints.org/8075/1/LT_HJS_Armicarb_7.4.06.pdf.
- ↑ "How to Toss Your Spanish Moss". SkyFrog landscape company. 4 December 2020. https://skyfroglandscape.com/spanish-moss-removal/.
- ↑ "saleratus". Merriam-Webster. https://www.merriam-webster.com/dictionary/saleratus.
External links
- Potassium Bicarbonate Handbook
- OMRI Potassium Bicarbonate
- Safety Data sheet - potassium bicarbonate
 "Saleratus". New International Encyclopedia. 1905.
"Saleratus". New International Encyclopedia. 1905.
| H2CO3 | He | ||||||||||||||||
| Li2CO3, LiHCO3 |
BeCO3 | B | C | (NH4)2CO3, NH4HCO3 |
O | F | Ne | ||||||||||
| Na2CO3, NaHCO3, Na3H(CO3)2 |
MgCO3, Mg(HCO3)2 |
Al2(CO3)3 | Si | P | S | Cl | Ar | ||||||||||
| K2CO3, KHCO3 |
CaCO3, Ca(HCO3)2 |
Sc | Ti | V | Cr | MnCO3 | FeCO3 | CoCO3 | NiCO3 | CuCO3 | ZnCO3 | Ga | Ge | As | Se | Br | Kr |
| Rb2CO3 | SrCO3 | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2CO3 | CdCO3 | In | Sn | Sb | Te | I | Xe |
| Cs2CO3, CsHCO3 |
BaCO3 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl2CO3 | PbCO3 | (BiO)2CO3 | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La2(CO3)3 | Ce2(CO3)3 | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UO2CO3 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |



