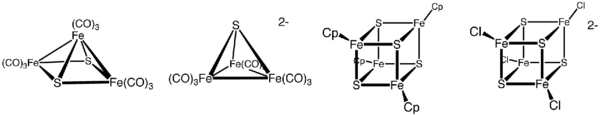Chemistry:Iron–sulfur cluster
Iron–sulfur clusters are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron–sulfur proteins, which are pervasive.[2] Many Fe–S clusters are known in the area of organometallic chemistry and as precursors to synthetic analogues of the biological clusters (see Figure). It is believed that the last universal common ancestor had many iron-sulfur clusters.[3]
Organometallic clusters
Organometallic Fe–S clusters include the sulfido carbonyls with the formula Fe2S2(CO)6, H2Fe3S(CO)9, and Fe3S2(CO)9. Compounds are also known that incorporate cyclopentadienyl ligands, such as (C5H5)4Fe4S4.[4]
Inorganic materials
thumb|center|Structure of potassium dithioferrate, which features infinite chains of Fe(III) centers.
Biological Fe–S clusters
Iron–sulfur clusters occur in many biological systems, often as components of electron transfer proteins. The ferredoxin proteins are the most common Fe–S clusters in nature. They feature either 2Fe–2S or 4Fe–4S centers. They occur in all branches of life.[5]
Fe–S clusters can be classified according to their Fe:S stoichiometry [2Fe–2S], [4Fe–3S], [3Fe–4S], and [4Fe–4S].[6] The [4Fe–4S] clusters occur in two forms: normal ferredoxins and high potential iron proteins (HiPIP). Both adopt cuboidal structures, but they utilize different oxidation states. They are found in all forms of life.[7]
The relevant redox couple in all Fe–S proteins is Fe(II)/Fe(III).[7]
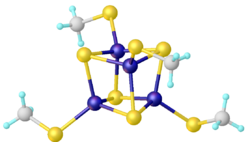
Many clusters have been synthesized in the laboratory with the formula [Fe4S4(SR)4]2−, which are known for many R substituents, and with many cations. Variations have been prepared including the incomplete cubanes [Fe3S4(SR)3]3−.[8]
See also
References
- ↑ Axel Kern; Christian Näther; Felix Studt; Felix Tuczek (2004). "Application of a Universal Force Field to Mixed Fe/Mo−S/Se Cubane and Heterocubane Clusters. 1. Substitution of Sulfur by Selenium in the Series [Fe4X4(YCH3)4]2-; X = S/Se and Y = S/Se". Inorg. Chem. 43 (16): 5003–5010. doi:10.1021/ic030347d. PMID 15285677.
- ↑ S. J. Lippard, J. M. Berg "Principles of Bioinorganic Chemistry" University Science Books: Mill Valley, CA; 1994. ISBN:0-935702-73-3.
- ↑ Weiss, Madeline C., et al. "The physiology and habitat of the last universal common ancestor." Nature microbiology 1.9 (2016): 1-8.
- ↑ Ogino, H.; Inomata, S.; Tobita, H. (1998). "Abiological Iron-Sulfur Clusters". Chem. Rev. 98 (6): 2093–2122. doi:10.1021/cr940081f. PMID 11848961.
- ↑ Johnson, D. C.; Dean, D. R.; Smith, A. D.; Johnson, M. K. (2005). "Structure, function, and formation of biological iron-sulfur clusters". Annual Review of Biochemistry 74: 247–281. doi:10.1146/annurev.biochem.74.082803.133518. PMID 15952888.
- ↑ Lill, Roland (2015). "Issue of iron-sulfur protein". Biochimica et Biophysica Acta 1853 (6): 1251–1252. doi:10.1016/j.bbamcr.2015.03.001. PMID 25746719.
- ↑ 7.0 7.1 Fisher, N (1998). "Intramolecular electron transfer in [4Fe–4S)]". The EMBO Journal: 849–858.
- ↑ Rao, P. V.; Holm, R. H. (2004). "Synthetic Analogues of the Active Sites of Iron-Sulfur Proteins". Chem. Rev. 104 (2): 527─559. doi:10.1021/Cr020615+. PMID 14871134.
External links
 |