Biology:Betaine—homocysteine S-methyltransferase
| betaine-homocysteine S-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
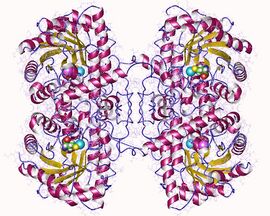
 Crystal structure of rat liver betaine homocysteine s-methyltransferase.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 2.1.1.5 | ||||||||
| CAS number | 9029-78-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
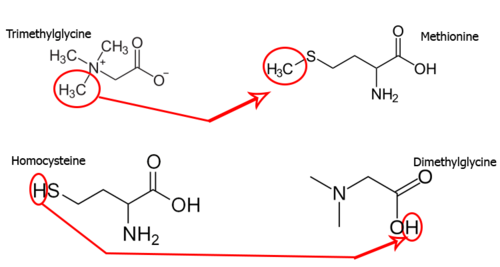
In the field of enzymology, a betaine-homocysteine S-methyltransferase also known as betaine-homocysteine methyltransferase (BHMT) is a zinc metallo-enzyme that catalyzes the transfer of a methyl group from trimethylglycine and a hydrogen ion from homocysteine to produce dimethylglycine and methionine respectively:[2]
- Trimethylglycine (methyl donor) + homocysteine (hydrogen donor) → dimethylglycine (hydrogen receiver) + methionine (methyl receiver)
This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. This enzyme participates in the metabolism of glycine, serine, threonine and also methionine.
Isozymes
In humans, there are two isozymes, BHMT[3][4] and BHMT2,[5][6] each encoded by a separate gene.
|
| ||||||||||||||||||||||||||||||||||||||||||||||
Tissue distribution
BHMT is expressed most predominantly in the liver and kidney.[7]
Clinical significance
Mutations in the BHMT gene are known to exist in humans. Anomalies may influence the metabolism of homocysteine , which is implicated in disorders ranging from vascular disease, autism, and schizophrenia to neural tube birth defects such as spina bifida.
See also
References
- ↑ PDB: 1UMY; "Crystal structure of rat liver betaine homocysteine s-methyltransferase reveals new oligomerization features and conformational changes upon substrate binding". J. Mol. Biol. 338 (4): 771–82. May 2004. doi:10.1016/j.jmb.2004.03.005. PMID 15099744.
- ↑ "Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism?". Cell. Mol. Life Sci. 63 (23): 2792–803. December 2006. doi:10.1007/s00018-006-6249-6. PMID 17086380.
- ↑ Garrow TA (September 1996). "Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase". J. Biol. Chem. 271 (37): 22831–8. doi:10.1074/jbc.271.37.22831. PMID 8798461.
- ↑ "Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene". Arch. Biochem. Biophys. 345 (1): 171–4. September 1997. doi:10.1006/abbi.1997.0246. PMID 9281325.
- ↑ "Betaine-homocysteine methyltransferase-2: cDNA cloning, gene sequence, physical mapping, and expression of the human and mouse genes". Genomics 70 (1): 66–73. November 2000. doi:10.1006/geno.2000.6319. PMID 11087663.
- ↑ "Betaine-homocysteine S-methyltransferase-2 is an S-methylmethionine-homocysteine methyltransferase". J. Biol. Chem. 283 (14): 8939–45. April 2008. doi:10.1074/jbc.M710449200. PMID 18230605.
- ↑ "Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene". Arch. Biochem. Biophys. 345 (1): 171–4. September 1997. doi:10.1006/abbi.1997.0246. PMID 9281325.
Further reading
- "The synthesis of methionine by enzymic transmethylation. VII Existence of two separate homocysteine methylpherases on mammalian liver". Biochim. Biophys. Acta 54: 157–64. 1961. doi:10.1016/0006-3002(61)90948-9. PMID 14456704.
External links
- Betaine+Homocysteine+Methyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)
- EC 2.1.1.5
 |