Chemistry:Levomefolic acid

| |
| Names | |
|---|---|
| IUPAC name
(2S)-2-[ [4-[(2-Amino-5-methyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl) methylamino]benzoyl]amino]pentanedioic acid
| |
| Other names
(L-5-Me-THFA, L-5-Me-H4FA),
anion: L-5-methyltetrahydrofolate (L-5-Me-THF, L-5-Me-H4F), L-methylfolate Metafolin | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | 5-methyltetrahydrofolate |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C20H25N7O6 | |
| Molar mass | 459.463 g·mol−1 |
| Pharmacology | |
| 1=ATC code }} | B03BB51 (WHO) |
| oral, transdermal, subcutaneous | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
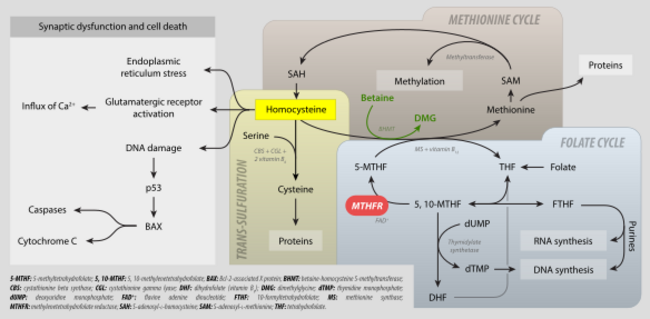
Levomefolic acid (INN, also known as L-5-MTHF, L-methylfolate and L-5-methyltetrahydrofolate and (6S)-5-methyltetrahydrofolate, and (6S)-5-MTHF) is the primary biologically active form of folate used at the cellular level for DNA reproduction, the cysteine cycle and the regulation of homocysteine. It is also the form found in circulation and transported across membranes into tissues and across the blood–brain barrier. In the cell, L-methylfolate is used in the methylation of homocysteine to form methionine and tetrahydrofolate (THF). THF is the immediate acceptor of one carbon unit for the synthesis of thymidine-DNA, purines (RNA and DNA) and methionine. The un-methylated form, folic acid (vitamin B9), is a synthetic form of folate, and must undergo enzymatic reduction by dihydrofolate reductase (DHFR) to become biologically active.[1]
It is synthesized in the absorptive cells of the small intestine from polyglutamylated dietary folate. It is a methylated derivative of tetrahydrofolate.
Levomefolic acid is generated by methylenetetrahydrofolate reductase (MTHFR) from 5,10-methylenetetrahydrofolate (MTHF) and used to recycle homocysteine back to methionine by methionine synthase (MS).[2]
L-Methylfolate is water-soluble and primarily excreted via the kidneys. In a study of 21 subjects with coronary artery disease, peak plasma levels were reached in one to three hours following oral or parenteral administration. Peak concentrations were found to be more than seven times higher than folic acid (129 ng/ml vs. 14.1 ng/ml).[3]
Metabolism
Medical uses
Major depressive disorder
Research suggests that levomefolic acid (L-methylfolate) taken with a first-line antidepressant[4] provides a modest adjunctive antidepressant effect for individuals who do not respond or have only a partial therapeutic response to SSRI or SNRI medication,[5][6] and might be a more cost-effective adjunctive agent than second-generation antipsychotics.[7]
Cardiovascular disease and cancer
Levomefolic acid (and folic acid in turn) has been proposed for treatment of cardiovascular disease[8][9] and advanced cancers such as breast and colorectal cancers.[10] It bypasses several metabolic steps in the body and better binds thymidylate synthase with FdUMP, a metabolite of the drug fluorouracil.
Patent issues
In March 2012, Merck & Cie of Switzerland , Pamlab LLC (maker of Metanx and Cerefolin, Neevo DHA, and Deplin), and South Alabama Medical Science Foundation (SAMSF) (the plaintiffs) filed a complaint in the United States District Court for the Eastern District of Texas against four defendants: Macoven Pharmaceuticals (owned by Pernix Therapeutics), Gnosis SpA of Italy, Gnosis U.S.A and Gnosis Bioresearch Switzerland. The plaintiffs alleged that the defendants infringed on several of the plaintiffs' patents.[11] The Macoven products named in the suit are: "Vitaciric-B", "ALZ-NAC", "PNV DHA", and l-methylfolate calcium (levomefolate calcium).[12]
In September 2012, the same three plaintiffs filed a complaint requesting that the International Trade Commission begin a investigation of the same four defendants. The complaint states that Gnosis' "Extrafolic-S" and products which are made from it, infringe upon three of their patents: US patent 5997915, US patent 6673381, and US patent 7172778.[13]
Formulations
Levomefolate calcium, a calcium salt of levomefolic acid is sold under the brand name Metafolin[14] and incorporated in Deplin.[15] Levomefolate magnesium is a magnesium salt of levomefolic acid, manufactured as DeltaFolate, a primary ingredient in EnLyte.[16]
See also
- 5,10-Methylenetetrahydrofolate
- S-Adenosylmethionine (SAMe)
References
- ↑ "Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics". Clinical Pharmacokinetics 49 (8): 535–48. August 2010. doi:10.2165/11532990-000000000-00000. PMID 20608755.
- ↑ "5-methyltetrahydrofolate – Compound Summary", PubChem (NCBI), https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?q=all&cid=444412#ec, retrieved 2012-09-25
- ↑ "CerefolinNAC Caplets Package Insert". http://intetlab.com/site/products/Cerefolin-NAC_package-insert_%204-26-10.pdf.
- ↑ Sussman, Norman (1 March 2009). "Selecting a First-line Antidepressant: New Analysis". Primary Psychiatry 16: 19–22. http://primarypsychiatry.com/selecting-a-first-line-antidepressant-new-analysis/.
- ↑ Maruf, Abdullah Al; Poweleit, Ethan A.; Brown, Lisa C.; Strawn, Jeffrey R.; Bousman, Chad A. (2022). "Systematic Review and Meta-Analysis of L-Methylfolate Augmentation in Depressive Disorders". Pharmacopsychiatry 55 (3): 139–147. doi:10.1055/a-1681-2047. ISSN 1439-0795. PMID 34794190. https://pubmed.ncbi.nlm.nih.gov/34794190/.
- ↑ Lam, Nelson Siu Kei; Long, Xin Xin; Li, Xuegang; Saad, Mirette; Lim, Florence; Doery, James CG; Griffin, Robert C.; Galletly, Cherrie (2022). "The potential use of folate and its derivatives in treating psychiatric disorders: A systematic review" (in en). Biomedicine & Pharmacotherapy 146: 112541. doi:10.1016/j.biopha.2021.112541. https://linkinghub.elsevier.com/retrieve/pii/S0753332221013287.
- ↑ "Comparative assessment of adherence measures and resource use in SSRI/SNRI-treated patients with depression using second-generation antipsychotics or L-methylfolate as adjunctive therapy". Journal of Managed Care Pharmacy 20 (1): 76–85. January 2014. doi:10.18553/jmcp.2014.20.1.76. PMID 24372461.
- ↑ "Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease". British Journal of Pharmacology (Nature Publishing Group) 141 (5): 825–30. March 2004. doi:10.1038/sj.bjp.0705446. PMID 14769778.
- ↑ "5,10-Methylenetetrahydrofolate reductase genotype determines the plasma homocysteine-lowering effect of supplementation with 5-methyltetrahydrofolate or folic acid in healthy young women". The American Journal of Clinical Nutrition (American Society for Clinical Nutrition) 75 (2): 275–82. February 2002. doi:10.1093/ajcn/75.2.275. PMID 11815318. http://webcache.googleusercontent.com/search?q=cache:NriMrILGOoIJ:www.geno-type.com/ts/GenoType/pdf/Fohr.pdf&cd=6&hl=en&ct=clnk.
- ↑ "Folic acid and colorectal cancer prevention: molecular mechanisms and epidemiological evidence (Review)". International Journal of Oncology 26 (6): 1449–64. June 2005. doi:10.3892/ijo.26.6.1449. PMID 15870856.
- ↑ The six patents named were US patent 5997915, US patent 6011040, US patent 6254904, US patent 6673381, US patent 7674490 and US patent 7172778.
- ↑ "Pernix Therapeutics' Subsidiary Macoven Pharmaceuticals, LLC Named in Suit by Merck, Pamlab, L.L.C. and Others for Alleged". Bloomberg. 6 March 2012. https://www.bloomberg.com/apps/news?pid=conewsstory&tkr=MKGAF:US&sid=aVfdD6EGy6Nc.
- ↑ Schweibenz, Eric W. (2012-09-10). "SAMSF, Merck, and Pamlab File New 337 Complaint Regarding Certain Reduced Folate Nutraceutical Products and L-methylfolate Raw Ingredients Used Therein". Oblon, Spivak, McClelland, Maier & Neustadt, L.L.P.. http://www.oblon.com/samsf-merck-and-pamlab-file-new-337-complaint-regarding-certain-reduced-folate-nutraceutical-product.
- ↑ "Metafolin". https://www.emdmillipore.com/US/en/products/small-molecule-pharmaceuticals/bulk-api/folates/l-metafolin/Metafolin/okeb.qB.qsoAAAFp2m8bIm4h,nav. "Metafolin® is our manufactured calcium salt of L-5-methyltetrahydrofolic or L-methylfolate. ... The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the US and Canada."
- ↑ "DEPLIN®" (in en-US). https://www.deplin.com/hcp.
- ↑ "EnLyte with DeltaFolate". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e83b2dd3-a9a0-40bc-ade4-6cd4db433b06.
External links
- Calcium L5-methyltetrahydrofolate (L-5-MTHF-Ca)
- "Localized depletion: the key to colorectal cancer risk mediated by MTHFR genotype and folate?". Cancer Causes & Control 17 (8): 1005–16. October 2006. doi:10.1007/s10552-006-0051-5. PMID 16933051.
- "Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent". CNS Spectrums 12 (10): 739–44. October 2007. doi:10.1017/S1092852900015418. PMID 17934378.
 |

