Biology:21-Hydroxylase
| Steroid 21-hydroxylase | |
|---|---|
| EC number | ? |
| Alt. names | "Cytochrome P450, family 21, subfamily A, polypeptide 2", CYP21A2, CYP21, CYP21B,[1] P45021A2, cytochrome P450c21,[2][3][4] steroid 21-monooxygenase,[5] 21-hydroxylase, 21α-hydroxylase,[6][7] 21β-hydroxylase[8][9] |
| Gene Ontology | AmiGO / QuickGO |
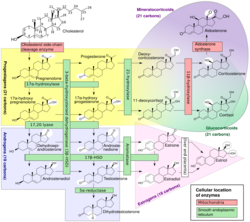
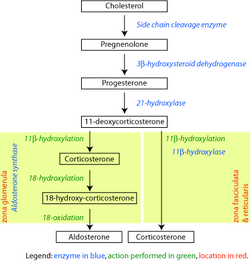
Steroid 21-hydroxylase is a protein that in humans is encoded by the CYP21A2 gene. The protein is an enzyme that hydroxylates steroids at the C21 position on the molecule.[10][11] Naming conventions for enzymes are based on the substrate acted upon and the chemical process performed. Biochemically, this enzyme is involved in the biosynthesis of the adrenal gland hormones aldosterone and cortisol, which are important in blood pressure regulation, sodium homeostasis and blood sugar control. The enzyme converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively,[12][13] within metabolic pathways which in humans ultimately lead to aldosterone and cortisol creation—deficiency in the enzyme may cause congenital adrenal hyperplasia.
Steroid 21-hydroxylase is a member of the cytochrome P450 family of monooxygenase enzymes that use an iron-containing heme cofactor to oxidize substrates.
In humans, the enzyme is localized in endoplasmic reticulum membranes of cells in adrenal cortex,[14][15] and is encoded by the CYP21A2 gene which is located near the CYP21A1P pseudogene that has high degree of sequence similarity. This similarity makes it difficult to analyze the gene at the molecular level, and sometimes leads to loss-of-function mutations of the gene due to intergenic exchange of DNA.
Gene
 Generic protein structure example |
Steroid 21-hydroxylase in humans is encoded by the CYP21A2 gene that may be accompanied by one or several copies of the nonfunctional pseudogene CYP21A1P,[16][17] this pseudogene shares 98% of the exonic informational identity with the actual functional gene.[18][19]
Pseudogenes are common in genomes, and they originate as artifacts during the duplication process. Though often thought of as "junk DNA", research has shown that retaining these faulty copies can have a beneficial role, often providing regulation of their parent genes.[20]
In the mouse genome, the Cyp21a2 is a pseudogene and the Cyp21a1 is a functional gene.[21] In the chicken and quail, there is only a single Cyp21 gene, which locus is located between complement component C4 and TNX gene, along with Cenpa.[22]
CYP21A2 in humans is located in chromosome 6, in the major histocompatibility complex III (MHC class III)[23] close to the Complement component 4 genes C4A and C4B, the Tenascin X gene TNXB and STK19.[24] MHC class III is the most gene-dense region of the human genome, containing many genes that have, as of 2023 - unknown functions or structures.[25][23]
Inside the MHC class III, CYP21A2 is located within the RCCX cluster (an abbreviation composed of the names of the genes RP (a former name for STK19 serine/threonine kinase 19),[26][27] C4, CYP21 and TNX),[28] which is the most complex gene cluster in the human genome.[29] The number of RCCX segments varies between one and four in a chromosome,[26] with the prevalence of approximately 15% for monomodular, 75% for bimodular (STK19-C4A-CYP21A1P-TNXA-STK19B-C4B-CYP21A2-TNXB),[27][30] and 10% for trimodular in Europeans.[31] The quadrimodular structure of the RCCX unit is very rare.[32][26][31] In a monomodular structure, all of the genes are functional i.e. protein-coding, but if a module count is two or more, there is only one copy of each functional gene rest being non-coding pseudogenes with the exception of the C4 gene which always has active copies.[26][31]
Due to the high degree of homology between the CYP21A2 gene and the CYP21A1P pseudogene and the complexity of the RCCX locus, it is difficult to perform molecular diagnostics for CYP21A2. The pseudogene can have single-nucleotide polymorphisms (SNP) that are identical or similar to those in the functional gene, making it difficult to distinguish between them. The pseudogene can also recombine with the functional gene, creating hybrid genes that have features of both. This can result in false-positive or false-negative results when testing for SNPs in the CYP21A2.[33]
The whole genome sequencing technology relies on breaking the DNA into small fragments, sequencing them, and then assembling them back together based on their overlaps. However, because of the high homology and variability of the CYP21A2 and its pseudogene, the fragments cannot be mapped unambiguously to either copy of the gene. This can lead to errors or gaps in the assembly, or missing some variants that are present in the gene.[34][33]
Polymerase chain reaction (PCR) molecular diagnostics uses selective primers to amplify specific segments of the DNA sequence that are relevant for diagnosing or detecting a certain disease or condition. If the primers are not designed carefully, they may bind to both the CYP21A2 and the CYP21A1P pseudogene, or to different segments of the RCCX cluster, resulting in false-positive or false-negative results. Therefore, PCR for the CYP21A2 requires the use of locus-specific primers that can distinguish between the gene and the pseudogene, and between different RCCX modules. Moreover, PCR may not be able to detect complex variants such as large gene conversions, deletions, or duplications, which are frequent in the case of the CYP21A2.[35][36][34]
Southern blotting, a method used for detecting and quantifying a specific DNA sequence in DNA samples, also has limitations in analyzing CYP21A2. This method is time-consuming and requires a large amount of good-quality DNA, which makes it less applicable in routine diagnostic settings. This method comes with a radioactive biohazard, which poses safety concerns and makes it labor-intensive. Southern blotting is unable to detect the junction sites of chimeras. The CYP21A2 gene is prone to mismatch and rearrangement, producing different types of complex variations that include copy number variants, large gene conversions, small insertions/deletions, and single-nucleotide (SNP) variants. Southern blotting is not capable of detecting all these types of variants simultaneously. Besides that, southern blotting requires genetic analysis of the parents, which is not always feasible or practical.[34][37]
Therefore, to analyze the CYP21A2 gene accurately, a more specialized and sensitive method is needed, such as targeted long-read sequencing, which can sequence longer DNA fragments and capture more information about the gene structure and variation. However, this method is not widely available or affordable for clinical use.[38][39][40]
Protein
Steroid 21-hydroxylase, is a member of the cytochrome P450 family of monooxygenase enzymes, the protein has 494 amino acid residues with a molecular weight of 55,000. This enzyme is at most 28% homologous to other P-450 enzymes that have been studied.[41]
Structurally, the protein contains an evolutionarily conserved core of four α-helix bundles (the importance of such genetic conservation is in demonstrating the functional importance of this aspect of this protein's structure). In addition, it has two additional alpha helices, two sets of β-sheets, and a heme cofactor binding loop.[42] Each subunit in the human enzyme consists of a total of 13 α-helices and 9 β-strands that folds into a triangular prism-like tertiary structure.[12]
The iron(III) heme group that defines the active site resides in the center of each subunit. The human enzyme binds one substrate at a time.[12] In contrast, the well-characterized bovine enzyme can bind two substrates.[43] The human and bovine enzyme share 80% amino acid sequence identity, but are structurally different, particularly in loop regions, and also evident in secondary structure elements.[12]
Species
Variations of the steroid 21-hydroxylase can be found in all vertebrates.[44]
Cyp21 first emerged in chordates before the speciation between basal chordates and vertebrates.[45] The sea lamprey, an early jawless fish species that originated over 500 million years ago, provides valuable insights into the evolution and emergence of Cyp21. Sea lampreys lack the 11β-hydroxylase enzyme responsible for converting 11-deoxycortisol to cortisol as observed in mammals. Instead, they rely on 11-deoxycortisol, a product of a reaction catalyzed by CYP21, as their primary glucocorticoid hormone with mineralocorticoid properties. This suggests the presence of a complex and highly specific corticosteroid signaling pathway that emerged at least half a billion years ago during early vertebrate evolution.[46]
In vertebrates, such as fish, amphibians, reptiles, birds, and mammals, Cyp21 participates in the biosynthesis of glucocorticoids and mineralocorticoids, therefore, Cyp21 is essential for the regulation of stress response, electrolyte balance and blood pressure, immune system, and metabolism in vertebrates.[47]
Cyp21 is relatively conserved among mammals, and shows some variations in its structure, expression, and regulation.[47] Rhesus macaques and orangutans possess two copies of Cyp21, while chimpanzees have three, still, a pseudogene (CYP21A1P) is only present in humans among primates.[48]
Tissue and subcellular distribution
Steroid 21-hydroxylase is localized in microsomes of endoplasmic reticulum membranes within adrenal cortex.[10] It is one of three microsomal steroidogenic cytochrome P450 enzymes, the others being steroid 17-hydroxylase and aromatase.[49]
Unlike other enzymes of the cytochrome P450 superfamily of enzymes that are expressed in multiple tissues, with most abundant expression in the liver, in adult humans steroid 21-hydroxylase, along with steroid 11β-hydroxylase and aldosterone synthase, is almost exclusively expressed in the adrenal gland.[50][51]
(As of 2023) the main subcellular location for the encoded protein in human cells is not known, and is pending cell analysis.[52]
Function
The enzyme, steroid 21-hydroxylase hydroxylates steroids at the C21 position.[13]Steroids are a group of naturally occurring and synthetically produced organic compounds, steroids all share a four ring primary structure. The enzyme catalyzes the chemical reaction in which the hydroxyl group (-OH) is added at the C21 position of the steroid biomolecule. This location is on a side chain of the D ring.
The enzyme is a member of the cytochrome P450 superfamily of monooxygenase enzymes. The cytochrome P450 enzymes catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids.
Steroid 21-hydroxylase is essential for the biosynthesis of cortisol and aldosterone.[53][54]
Mechanism
Steroid 21-hydroxylase is a cytochrome P450 enzyme that is notable for its substrate specificity and relatively high catalytic efficiency.[44]
Like other cytochrome P450 enzymes, steroid 21-hydroxylase participates in the cytochrome P450 catalytic cycle and engages in one-electron transfer with NADPH-P450 reductase. Steroid 21-hydroxylase is highly specific for hydroxylation of progesterone and 17-hydroxyprogesterone. This is in marked contrast to the evolutionarily and functionally related P450 enzyme 17-hydroxylase, which has a broad range of substrates.[55]
The chemical reaction in which steroid 21-hydroxylase catalyzes the addition of hydroxyl (-OH) to the C21 position of progesterone, 17α-hydroxyprogesterone and 21-desoxycortisone[56] was first described in 1952.[57]
Studies of the human enzyme expressed in yeast initially classified 17-hydroxyprogesterone as the preferred substrate for steroid 21-hydroxylase,[55][58][59] however, later analysis of the purified human enzyme found a lower KM and greater catalytic efficiency for progesterone over 17-hydroxyprogesterone.[12]
The catalytic efficiency of steroid 21-hydroxylase for conversion of progesterone in humans is approximately 1.3 x 107 M−1s−1 at 37 °C. This makes it the most catalytically efficient P450 enzyme of those reported to date, and catalytically more efficient than the closely related bovine steroid 21-hydroxylase enzyme.[14] C-H bond breaking to create a primary carbon radical is thought to be the rate-limiting step in the hydroxylation.[12]
Clinical significance
Congenital adrenal hyperplasia
Genetic variants in the CYP21A2 gene cause a disturbance in the development of the enzyme, leading to congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Gene conversion events involving the functional gene and the pseudogene account for many cases of steroid 21-hydroxylase deficiency.[60] CAH is an autosomal recessive disorder. There are multiple forms of CAH, defined as classical and nonclassical forms based on the amount of enzyme function still present in the patient.
The classical forms occur in approximately 1 in 10000 to 1 in 20000 births globally,[54][61] and includes both the salt-wasting (excessive excretion of sodium via the urine causing hyponatremia and dehydration) and simple-virilizing forms. Complete loss of enzymatic activity causes the salt-wasting form. Variations in the structure of steroid 21-hydroxylase are related to the clinical severity of congenital adrenal hyperplasia. Cortisol and aldosterone deficits are associated with life-threatening sodium loss, as the steroids play roles in regulating sodium homeostasis. Simple-virilizing CAH patients (~1-2% enzyme function)[54] maintain adequate sodium homeostasis, but exhibit other symptoms shared by the salt-wasting form, including accelerated growth in childhood and ambiguous genitalia in female neonates.
The nonclassical form is the mildest condition, retaining about 20% to 50% of enzyme function.[54] This form is associated with mild and clinically silent cortisol impairment,[61] but an excess of androgens post-puberty.[62]
Non-classic congenital adrenal hyperplasia
Women with NCCAH usually have normal female genitalia at birth. In later life, the signs and symptoms of the condition may include acne, hirsutism, male-pattern baldness, irregular menstruation, and infertility.[54][61][21]
Fewer studies have been published about males with NCCAH comparing to those about females, because males are generally asymptomatic.[21][54] Males, however, may present with acne[63][64] and early balding.[65][66]
While symptoms are usually diagnosed after puberty, children may present with premature adrenarche.[67]
Research on other conditions
There is ongoing research on how Genetic variants in the CYP21A2 gene may lead to pathogenic conditions. A variant of this gene has been reported to cause autosomal dominant posterior polar cataract, suggesting that steroid 21-hydroxylase may be involved in the extra-adrenal biosynthesis of aldosterone and cortisol in the lens of the eye.[68]
History
In the 1950s and 1960s, steroidogenic pathways that included cholesterol conversion to progesterone through a complex pathway involving multiple steps were identified, and, among them, a pathway for cortisol synthesis showing specific enzymatic steps that included hydroxylation reactions at position 21 (21-hydroxylation) mediated by cytochrome P450 enzymes.[69] Cytochrome P450 enzymes were then described, and steroid 21-hydroxylation was associated with cytochrome P450.[70][69]
In the 1980s and 1990s, partial-length bovine Cyp21 cDNA clones were identified as related to human CYP21A2.[71][69] Researchers discovered mutations in the CYP21A2 gene associated with congenital adrenal hyperplasia (CAH).[69]
From the 1990s onward, specific mutations were correlated with different forms/severity levels of CAH. Genotype/phenotype correlations were investigated for improved diagnostic accuracy.[69]
See also
References
- ↑ "UniProt". https://www.uniprot.org/uniprotkb/P08686/entry.
- ↑ "Molecular analysis of the CYP21A2 gene in dried blood spot samples". Medicina 80 (3): 197–202. 2020. PMID 32442933.
- ↑ "Expression level of the cytochrome P450c21 (CYP21) protein correlating to drip loss in pigs". Animal Science Journal 88 (11): 1855–1859. November 2017. doi:10.1111/asj.12863. PMID 28677294.
- ↑ "Why human cytochrome P450c21 is a progesterone 21-hydroxylase". Biochemistry 50 (19): 3968–74. May 2011. doi:10.1021/bi102078e. PMID 21446712.
- ↑ "Information on EC 1.14.14.16 - steroid 21-monooxygenase - BRENDA Enzyme Database". https://www.brenda-enzymes.org/enzyme.php?ecno=1.14.14.16.
- ↑ "Expression of genes encoding mineralocorticoid biosynthetic enzymes and the mineralocorticoid receptor, and levels of mineralocorticoids in the bovine follicle and corpus luteum". The Journal of Reproduction and Development 66 (1): 75–81. February 2020. doi:10.1262/jrd.2019-127. PMID 31839646.
- ↑ "Molecular testing in congenital adrenal hyperplasia due to 21α-hydroxylase deficiency in the era of newborn screening". Clinical Genetics 82 (1): 64–70. July 2012. doi:10.1111/j.1399-0004.2011.01694.x. PMID 21534945.
- ↑ "Brain white matter impairment in congenital adrenal hyperplasia". Archives of Neurology 63 (3): 413–6. March 2006. doi:10.1001/archneur.63.3.413. PMID 16540460.
- ↑ "Congenital adrenal hyperplasia conditioned by 21beta-hydroxylase deficiency - clinical considerations" (in pl). Endokrynologia, Diabetologia I Choroby Przemiany Materii Wieku Rozwojowego 6 (1): 67–9. 2000. PMID 14640134.
- ↑ 10.0 10.1 Template:NCBI RefSeq
- ↑ "Hydroxylation of steroids at carbon 21". The Journal of Biological Chemistry 225 (1): 103–14. March 1957. doi:10.1016/S0021-9258(18)64913-0. PMID 13416221. http://www.jbc.org/content/225/1/103.full.pdf.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 "Human Cytochrome P450 21A2, the Major Steroid 21-Hydroxylase: structure of the enzyme progesterone substrate complex and rate-limiting c-h bond cleavage". The Journal of Biological Chemistry 290 (21): 13128–43. May 2015. doi:10.1074/jbc.M115.646307. PMID 25855791.
- ↑ 13.0 13.1 "The steroid metabolite 16(β)-OH-androstenedione generated by CYP21A2 serves as a substrate for CYP19A1". The Journal of Steroid Biochemistry and Molecular Biology 167: 182–191. March 2017. doi:10.1016/j.jsbmb.2017.01.002. PMID 28065637.
- ↑ 14.0 14.1 "Recent Structural Insights into Cytochrome P450 Function". Trends in Pharmacological Sciences 37 (8): 625–40. August 2016. doi:10.1016/j.tips.2016.05.006. PMID 27267697.
- ↑ "Mechanism of intermolecular interactions of microsomal cytochrome P450s CYP17 and CYP21 involved in steroid hormone biosynthesis". Biochemistry. Biokhimiia 77 (6): 585–92. June 2012. doi:10.1134/S0006297912060041. PMID 22817457.
- ↑ Template:NCBI RefSeq
- ↑ "EMQN best practice guidelines for molecular genetic testing and reporting of 21-hydroxylase deficiency". European Journal of Human Genetics 28 (10): 1341–1367. October 2020. doi:10.1038/s41431-020-0653-5. PMID 32616876.
- ↑ "Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene". Proceedings of the National Academy of Sciences of the United States of America 83 (9): 2841–5. May 1986. doi:10.1073/pnas.83.9.2841. PMID 3486422. Bibcode: 1986PNAS...83.2841H.
- ↑ "CYP21A2 intronic variants causing 21-hydroxylase deficiency". Metabolism: Clinical and Experimental 71: 46–51. June 2017. doi:10.1016/j.metabol.2017.03.003. PMID 28521877.
- ↑ Biochemistry, Pseudogenes. Treasure Island (FL): StatPearls Publishing. 2023. Template:NCBIBook.
- ↑ 21.0 21.1 21.2 "Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells". Proceedings of the National Academy of Sciences of the United States of America 82 (23): 7860–4. December 1985. doi:10.1073/pnas.82.23.7860. PMID 2999780. Bibcode: 1985PNAS...82.7860P.
- ↑ "Comparative genomic analysis of two avian (quail and chicken) MHC regions". Journal of Immunology 172 (11): 6751–63. June 2004. doi:10.4049/jimmunol.172.11.6751. PMID 15153492.
- ↑ 23.0 23.1 "Molecular genetics of the human MHC complement gene cluster". Experimental and Clinical Immunogenetics 15 (4): 213–230. 1999. doi:10.1159/000019075. PMID 10072631.
- ↑ "Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man". Proceedings of the National Academy of Sciences of the United States of America 82 (4): 1089–1093. February 1985. doi:10.1073/pnas.82.4.1089. PMID 2983330. Bibcode: 1985PNAS...82.1089W.
- ↑ "Analysis of the gene-dense major histocompatibility complex class III region and its comparison to mouse". Genome Research 13 (12): 2621–2636. December 2003. doi:10.1101/gr.1736803. PMID 14656967.
- ↑ 26.0 26.1 26.2 26.3 "Fine-tuned characterization of RCCX copy number variants and their relationship with extended MHC haplotypes". Genes and Immunity 13 (7): 530–535. October 2012. doi:10.1038/gene.2012.29. PMID 22785613.
- ↑ 27.0 27.1 "Genes and Pseudogenes: Complexity of the RCCX Locus and Disease". Frontiers in Endocrinology 12: 709758. 2021. doi:10.3389/fendo.2021.709758. PMID 34394006.
- ↑ "C4B null alleles are not associated with genetic polymorphisms in the adjacent gene CYP21A2 in autism". BMC Medical Genetics 9: 1. January 2008. doi:10.1186/1471-2350-9-1. PMID 18179706.
- ↑ "Genetic organization of the human MHC class III region". Frontiers in Bioscience 6: D914–D926. August 2001. doi:10.2741/milner. PMID 11487476.
- ↑ "Molecular basis and genetic testing strategies for diagnosing 21-hydroxylase deficiency, including CAH-X syndrome". Annals of Pediatric Endocrinology & Metabolism 28 (2): 77–86. June 2023. doi:10.6065/apem.2346108.054. PMID 37401054.
- ↑ 31.0 31.1 31.2 "Intraspecific evolution of human RCCX copy number variation traced by haplotypes of the CYP21A2 gene". Genome Biol Evol 5 (1): 98–112. 2013. doi:10.1093/gbe/evs121. PMID 23241443.
- ↑ "Analysis of CYP21A1P and the duplicated CYP21A2 genes". Gene 506 (1): 261–262. September 2012. doi:10.1016/j.gene.2012.06.045. PMID 22771554.
- ↑ 33.0 33.1 "Molecular Diagnosis of Steroid 21-Hydroxylase Deficiency: A Practical Approach". Front Endocrinol 13: 834549. 2022. doi:10.3389/fendo.2022.834549. PMID 35422767.
- ↑ 34.0 34.1 34.2 "Challenges of CYP21A2 genotyping in children with 21-hydroxylase deficiency: determination of genotype-phenotype correlation using next generation sequencing in Southeastern Anatolia". J Endocrinol Invest 44 (11): 2395–2405. November 2021. doi:10.1007/s40618-021-01546-z. PMID 33677812.
- ↑ "Comprehensive mutation analysis of the CYP21A2 gene: an efficient multistep approach to the molecular diagnosis of congenital adrenal hyperplasia". J Mol Diagn 15 (6): 745–53. November 2013. doi:10.1016/j.jmoldx.2013.06.001. PMID 24071710.
- ↑ "Polymerase Chain Reaction (PCR) Fact Sheet". https://www.genome.gov/about-genomics/fact-sheets/Polymerase-Chain-Reaction-Fact-Sheet.
- ↑ "Southern blotting — Knowledge Hub". https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/southern-blotting/.
- ↑ "Corrigendum: Getting pregnant with congenital adrenal hyperplasia: assisted reproduction and pregnancy complications. A systematic review and meta-analysis". Front Endocrinol 14: 1269711. 2023. doi:10.3389/fendo.2023.1269711. PMID 37842302.
- ↑ "A MinION-Based Long-Read Sequencing Application with One-Step PCR for the Genetic Diagnosis of 21-Hydroxylase Deficiency". J Clin Endocrinol Metab. October 2023. doi:10.1210/clinem/dgad577. PMID 37804107.
- ↑ "Targeted long-read sequencing for comprehensive detection of CYP21A2 mutations in patients with 21-hydroxylase deficiency". Journal of Endocrinological Investigation. 2023. doi:10.1007/s40618-023-02197-y. PMID 37815751.
- ↑ "Steroid 21-hydroxylase | DrugBank Online" (in en). https://go.drugbank.com/polypeptides/P08686.
- ↑ "Cytochromes P450: a success story". Genome Biology 1 (6): REVIEWS3003. 2000. doi:10.1186/gb-2000-1-6-reviews3003. PMID 11178272.
- ↑ "Three-dimensional structure of steroid 21-hydroxylase (cytochrome P450 21A2) with two substrates reveals locations of disease-associated variants". The Journal of Biological Chemistry 287 (13): 10613–22. March 2012. doi:10.1074/jbc.M111.323501. PMID 22262854.
- ↑ 44.0 44.1 "Sequence alignments, variabilities, and vagaries". Cytochrome P450 Part C. Methods in Enzymology. 357. Academic Press. 2002. pp. 15–28. doi:10.1016/s0076-6879(02)57661-8. ISBN 9780121822606.
- ↑ "Origin of the response to adrenal and sex steroids: Roles of promiscuity and co-evolution of enzymes and steroid receptors". J Steroid Biochem Mol Biol 151: 12–24. July 2015. doi:10.1016/j.jsbmb.2014.10.020. PMID 25445914.
- ↑ "Corticosteroid control of Na+/K+-ATPase in the intestine of the sea lamprey (Petromyzon marinus)". General and Comparative Endocrinology 307: 113756. March 2021. doi:10.1016/j.ygcen.2021.113756. PMID 33741310.
- ↑ 47.0 47.1 "Conservation of the central MHC genome: PFGE mapping and RFLP analysis of complement, HSP70, and TNF genes in the goat". Immunogenetics 31 (4): 253–64. 1990. doi:10.1007/BF00204897. PMID 1970334.
- ↑ "Substrate-dependent evolution of cytochrome P450: rapid turnover of the detoxification-type and conservation of the biosynthesis-type". PLOS ONE 9 (6): e100059. 2014. doi:10.1371/journal.pone.0100059. PMID 24977709. Bibcode: 2014PLoSO...9j0059K.
- ↑ "P450 Enzymes in Steroid Processing". Cytochrome P450: Structure, Mechanism, and Biochemistry (Fourth ed.). Springer International Publishing. 2015. pp. 851–879. doi:10.1007/978-3-319-12108-6_12. ISBN 978-3-319-12107-9.
- ↑ "Enzyme Kinetics of Oxidative Metabolism: Cytochromes P450". Enzyme Kinetics in Drug Metabolism. Methods in Molecular Biology (Clifton, N.J.). 1113. Humana Press. 2014. pp. 149–166. doi:10.1007/978-1-62703-758-7_8. ISBN 978-1-62703-757-0.
- ↑ "Tissue expression of CYP21A2". The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000231852-CYP21A2/tissue.
- ↑ "CYP21A2 Subcellular RNA expression". The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000231852-CYP21A2/subcellular.
- ↑ "Microconversion between CYP21A2 and CYP21A1P promoter regions causes the nonclassical form of 21-hydroxylase deficiency". The Journal of Clinical Endocrinology and Metabolism 92 (10): 4028–34. October 2007. doi:10.1210/jc.2006-2163. PMID 17666484.
- ↑ 54.0 54.1 54.2 54.3 54.4 54.5 "Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline". The Journal of Clinical Endocrinology and Metabolism 103 (11): 4043–4088. November 2018. doi:10.1210/jc.2018-01865. PMID 30272171.
- ↑ 55.0 55.1 "The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21". Archives of Biochemistry and Biophysics 409 (1): 134–44. January 2003. doi:10.1016/s0003-9861(02)00491-5. PMID 12464252.
- ↑ "Irradiation and adrenal steroidogenesis: steroid transformations by irradiated isolated perfused calf adrenals". Endocrinology 56 (1): 24–9. 1955. doi:10.1210/endo-56-1-24. PMID 13220521.
- ↑ "The action of adrenal homogenates on progesterone, 17-hydroxyprogesterone and 21-desoxycortisone". Archives of Biochemistry and Biophysics 36 (1): 237–9. March 1952. doi:10.1016/0003-9861(52)90397-4. PMID 14934270.
- ↑ "Expression of a full-length cDNA encoding bovine adrenal cytochrome P450C21". Archives of Biochemistry and Biophysics 273 (1): 79–88. August 1989. doi:10.1016/0003-9861(89)90164-1. PMID 2502949.
- ↑ "Expression and functional study of wild-type and mutant human cytochrome P450c21 in Saccharomyces cerevisiae". DNA and Cell Biology 10 (3): 201–9. April 1991. doi:10.1089/dna.1991.10.201. PMID 1707279.
- ↑ "NCBI: CYP21A2 cytochrome P450 family 21 subfamily A member 2". National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/gene/1589. "This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases that catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids, and other lipids. This protein localizes to the endoplasmic reticulum and hydroxylates steroids at the 21 position. Its activity is required for the synthesis of steroid hormones including cortisol and aldosterone. Mutations in this gene cause congenital adrenal hyperplasia. A related pseudogene is located near this gene; gene conversion events involving the functional gene and the pseudogene are thought to account for many cases of steroid 21-hydroxylase deficiency. Two transcript variants encoding different isoforms have been found for this gene."
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 61.0 61.1 61.2 "Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency". The New England Journal of Medicine 383 (13): 1248–1261. September 2020. doi:10.1056/NEJMra1909786. PMID 32966723.
- ↑ "The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders". Endocrine Reviews 32 (1): 81–151. February 2011. doi:10.1210/er.2010-0013. PMID 21051590.
- ↑ "The frequency of 21-alpha hydroxylase enzyme deficiency and related sex hormones in Iraqi healthy male subjects versus patients with acne vulgaris". Saudi Medical Journal 30 (12): 1547–50. December 2009. PMID 19936418.
- ↑ "Nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency: clinical presentation, diagnosis, treatment, and outcome". Endocrine 50 (1): 32–50. September 2015. doi:10.1007/s12020-015-0656-0. PMID 26082286.
- ↑ "Extensive clinical experience: nonclassical 21-hydroxylase deficiency". The Journal of Clinical Endocrinology and Metabolism 91 (11): 4205–14. November 2006. doi:10.1210/jc.2006-1645. PMID 16912124. "Loss of scalp hair in females and males is embarrassing, requiring treatment with 5α-reductase inhibitors".
- ↑ Congenital Adrenal Hyperplasia: Diagnosis and Emergency Treatment. MDText.com. April 2019. https://www.ncbi.nlm.nih.gov/books/NBK279085/.
- ↑ "Nonclassic congenital adrenal hyperplasia". International Journal of Pediatric Endocrinology 2010: 625105. 2010. doi:10.1155/2010/625105. PMID 20671993.
- ↑ "Pathogenic variants in the CYP21A2 gene cause isolated autosomal dominant congenital posterior polar cataracts". Ophthalmic Genet 43 (2): 218–223. April 2022. doi:10.1080/13816810.2021.1998556. PMID 34748434.
- ↑ 69.0 69.1 69.2 69.3 69.4 "History of Adrenal Research: From Ancient Anatomy to Contemporary Molecular Biology". Endocr Rev 44 (1): 70–116. January 2023. doi:10.1210/endrev/bnac019. PMID 35947694.
- ↑ Fifty Years of Cytochrome P450 Research. 2014. doi:10.1007/978-4-431-54992-5. ISBN 978-4-431-54991-8.
- ↑ "Tenascin-X-Discovery and Early Research". Front Immunol 11: 612497. 2020. doi:10.3389/fimmu.2020.612497. PMID 33505400.
External links
- GeneReviews/NCBI/NIH/UW entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- OMIM entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- Synthesis of Desoxycorticosterone from Progesterone through 21-Hydroxylase (Image)
- Steroid+21-Hydroxylase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human CPS1 genome location and CPS1 gene details page in the UCSC Genome Browser.
- Human CYP21A2 genome location and CYP21A2 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P08686 (Steroid 21-hydroxylase) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |