Biology:N-Acetylglutamate synthase
| N-Acetylglutamate synthase | |
|---|---|
 N-Acetylglutamate synthase/kinase tetramer, Maricaulis maris | |
| Identifiers | |
| Symbol | NAGS |
| NCBI gene | 162417 |
| HGNC | 17996 |
| OMIM | 608300 |
| RefSeq | NM_153006 |
| UniProt | Q8N159 |
| Other data | |
| EC number | 2.3.1.1 |
| Locus | Chr. 17 q21.31 |
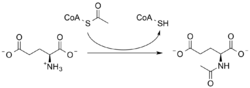
N-Acetylglutamate synthase (NAGS) is an enzyme that catalyses the production of N-acetylglutamate (NAG) from glutamate and acetyl-CoA.
Put simply NAGS catalyzes the following reaction:
- acetyl-CoA + L-glutamate → CoA + N-acetyl-L-glutamate
NAGS, a member of the N-acetyltransferase family of enzymes, is present in both prokaryotes and eukaryotes, although its role and structure differ widely depending on the species. NAG can be used in the production of ornithine and arginine, two important amino acids, or as an allosteric cofactor for carbamoyl phosphate synthase (CPS1). In mammals, NAGS is expressed primarily in the liver and small intestine, and is localized to the mitochondrial matrix.[1]
Biological function
Most prokaryotes (bacteria) and lower eukaryotes (fungus, green algae, plants, and so on) produce NAG through ornithine acetyltransferase (OAT), which is part of a ‘cyclic’ ornithine production pathway. NAGS is therefore used in a supportive role, replenishing NAG reserves as required. In some plants and bacteria, however, NAGS catalyzes the first step in a ‘linear’ arginine production pathway.[2]
The protein sequences of NAGS between prokaryotes, lower eukaryotes and higher eukaryotes have shown a remarkable lack of similarity. Sequence identity between prokaryotic and eukaryotic NAGS is largely <30%,[3] while sequence identity between lower and higher eukaryotes is ~20%.[4]
Enzyme activity of NAGS is modulated by L-arginine, which acts as an inhibitor in plant and bacterial NAGS, but an effector in vertebrates.[5][6] While the role of arginine as an inhibitor of NAG in ornithine and arginine synthesis is well understood, there is some controversy as to the role of NAG in the urea cycle.[7][8] The currently accepted role of NAG in vertebrates is as an essential allosteric cofactor for CPS1, and therefore it acts as the primary controller of flux through the urea cycle. In this role, feedback regulation from arginine would act to signal NAGS that ammonia is plentiful within the cell, and needs to be removed, accelerating NAGS function. As it stands, the evolutionary journey of NAGS from essential synthetic enzyme to primary urea cycle controller is yet to be fully understood.[9]
Mechanism
Two mechanisms for N-acetyltransferase function have been proposed: a two-step, ping-pong mechanism involving transfer of the relevant acetyl group to an activated cysteine residue[10] and a one-step mechanism through direct attack of the amino nitrogen on the carbonyl group.[11] Studies conducted using NAGS derived from Neisseria gonorrhoeae suggest that NAGS proceeds through the previously described one-step mechanism.[12] In this proposal, the carbonyl group of acetyl-CoA is attacked directly by the α-amino nitrogen of glutamate. This mechanism is supported by the activation of the carbonyl through hydrogen bond polarization, as well as the absence of a suitable cysteine within the active site to act as an intermediate acceptor of the acetyl group.[13][14]
Clinical significance
Inactivity of NAGS results in N-acetylglutamate synthase deficiency, a form of hyperammonemia.[15] In many vertebrates, N-acetylglutamate is an essential allosteric cofactor of CPS1, the enzyme that catalyzes the first step of the urea cycle.[16] Without NAG stimulation, CPS1 cannot convert ammonia to carbamoyl phosphate, resulting in toxic ammonia accumulation.[17] Carbamoyl glutamate has shown promise as a possible treatment for NAGS deficiency.[15] This is suspected to be a result of the structural similarities between NAG and carbamoyl glutamate, which allows carbamoyl glutamate to act as an effective agonist for CPS1.[14]
References
- ↑ "Control of ureogenesis". European Journal of Biochemistry 148 (1): 189–96. April 1985. doi:10.1111/j.1432-1033.1985.tb08824.x. PMID 3979393.
- ↑ "Biosynthesis and metabolism of arginine in bacteria". Microbiological Reviews 50 (3): 314–52. September 1986. doi:10.1128/MMBR.50.3.314-352.1986. PMID 3534538.
- ↑ "Acetylglutamate synthase from Neurospora crassa: structure and regulation of expression". Molecular Microbiology 22 (3): 545–54. November 1996. doi:10.1046/j.1365-2958.1996.1321494.x. PMID 8939437.
- ↑ "N-Acetylglutamate synthase: structure, function and defects". Molecular Genetics and Metabolism 100 (Suppl 1): S13–9. 2010. doi:10.1016/j.ymgme.2010.02.018. PMID 20303810.
- ↑ "Organization and control in the arginine biosynthetic pathway of Neurospora". Journal of Bacteriology 123 (1): 196–202. July 1975. doi:10.1128/JB.123.1.196-202.1975. PMID 166979.
- ↑ "Purification of N-acetyl-L-glutamate synthetase from rat liver mitochondria and substrate and activator specificity of the enzyme". The Journal of Biological Chemistry 258 (16): 9839–44. August 1983. doi:10.1016/S0021-9258(17)44574-1. PMID 6885773.
- ↑ "N-Acetylglutamate and urea synthesis". The Biochemical Journal 223 (2): 559–60. October 1984. doi:10.1042/bj2230559. PMID 6497864.
- ↑ "Is N-acetylglutamate a short-term regulator of urea synthesis?". The Biochemical Journal 218 (3): 991–4. March 1984. doi:10.1042/bj2180991. PMID 6721845.
- ↑ "N-Acetylglutamate and its changing role through evolution". The Biochemical Journal 372 (Pt 2): 279–90. June 2003. doi:10.1042/BJ20030002. PMID 12633501.
- ↑ "Kinetic mechanism of the reaction catalyzed by nuclear histone acetyltransferase from calf thymus". Biochemistry 22 (20): 4637–41. September 1983. doi:10.1021/bi00289a004. PMID 6626521.
- ↑ "GCN5-related N-acetyltransferases: a structural overview". Annual Review of Biophysics and Biomolecular Structure 29: 81–103. 2000. doi:10.1146/annurev.biophys.29.1.81. PMID 10940244.
- ↑ "The crystal structure of N-acetyl-L-glutamate synthase from Neisseria gonorrhoeae provides insights into mechanisms of catalysis and regulation". The Journal of Biological Chemistry 283 (11): 7176–84. March 2008. doi:10.1074/jbc.M707678200. PMID 18184660.
- ↑ "Mechanism of allosteric inhibition of N-acetyl-L-glutamate synthase by L-arginine". The Journal of Biological Chemistry 284 (8): 4873–80. February 2009. doi:10.1074/jbc.M805348200. PMID 19095660.
- ↑ 14.0 14.1 "Mammalian N-acetylglutamate synthase". Molecular Genetics and Metabolism 81 (Suppl 1): S4–11. April 2004. doi:10.1016/j.ymgme.2003.10.017. PMID 15050968.
- ↑ 15.0 15.1 "Null mutations in the N-acetylglutamate synthase gene associated with acute neonatal disease and hyperammonemia". Human Genetics 112 (4): 364–8. April 2003. doi:10.1007/s00439-003-0909-5. PMID 12594532.
- ↑ "Required allosteric effector site for N-acetylglutamate on carbamoyl-phosphate synthetase I". The Journal of Biological Chemistry 271 (30): 18285–94. July 1996. doi:10.1074/jbc.271.30.18285. PMID 8663466.
- ↑ "Restoration of ureagenesis in N-acetylglutamate synthase deficiency by N-carbamylglutamate". The Journal of Pediatrics 145 (4): 552–4. October 2004. doi:10.1016/j.jpeds.2004.06.047. PMID 15480384.
External links
- GeneReviews/NCBI/NIH/UW entry on Urea Cycle Disorders Overview
- N-Acetylglutamate+Synthase at the US National Library of Medicine Medical Subject Headings (MeSH)
 |