Biology:Quantum biology
Quantum biology is the study of applications of quantum mechanics and theoretical chemistry to biological objects and problems. Many biological processes involve the conversion of energy into forms that are usable for chemical transformations, and are quantum mechanical in nature. Such processes involve chemical reactions, light absorption, formation of excited electronic states, transfer of excitation energy, and the transfer of electrons and protons (hydrogen ions) in chemical processes, such as photosynthesis, olfaction and cellular respiration.[1]
Quantum biology may use computations to model biological interactions in light of quantum mechanical effects.[2] Quantum biology is concerned with the influence of non-trivial quantum phenomena,[3] which can be explained by reducing the biological process to fundamental physics, although these effects are difficult to study and can be speculative.[4]
History
Quantum biology is an emerging field; most of the current research is theoretical and subject to questions that require further experimentation. Though the field has only recently received an influx of attention, it has been conceptualized by physicists throughout the 20th century. It has been suggested that quantum biology might play a critical role in the future of the medical world.[5] Early pioneers of quantum physics saw applications of quantum mechanics in biological problems. Erwin Schrödinger's 1944 book What is Life? discussed applications of quantum mechanics in biology.[6] Schrödinger introduced the idea of an "aperiodic crystal" that contained genetic information in its configuration of covalent chemical bonds. He further suggested that mutations are introduced by "quantum leaps". Other pioneers Niels Bohr, Pascual Jordan, and Max Delbruck argued that the quantum idea of complementarity was fundamental to the life sciences.[7] In 1963, Per-Olov Löwdin published proton tunneling as another mechanism for DNA mutation. In his paper, he stated that there is a new field of study called "quantum biology".[8] In 1979, the Soviet and Ukrainian physicist Alexander Davydov published the first textbook on quantum biology entitled "Biology and Quantum Mechanics".[9][10]
Applications
Photosynthesis
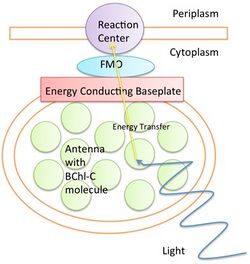
Organisms that undergo photosynthesis absorb light energy through the process of electron excitation in antennae. These antennae vary among organisms. For example, bacteria use ring-like antennae, while plants use chlorophyll pigments to absorb photons. Photosynthesis creates Frenkel excitons, which provide a separation of charge that cells convert into usable chemical energy. The energy collected in reaction sites must be transferred quickly before it is lost to fluorescence or thermal vibrational motion.
Various structures, such as the FMO complex in green sulfur bacteria, are responsible for transferring energy from antennae to a reaction site. FT electron spectroscopy studies of electron absorption and transfer show an efficiency of above 99%,[11] which cannot be explained by classical mechanical models like the diffusion model. Instead, as early as 1938, scientists theorized that quantum coherence was the mechanism for excitation energy transfer.
Scientists have recently looked for experimental evidence of this proposed energy transfer mechanism. A study published in 2007 claimed the identification of electronic quantum coherence[12] at −196 °C (77 K). Another theoretical study from 2010 provided evidence that quantum coherence lives as long as 300 femtoseconds at biologically relevant temperatures (4 °C or 277 K). In that same year, experiments conducted on photosynthetic cryptophyte algae using two-dimensional photon echo spectroscopy yielded further confirmation for long-term quantum coherence.[13] These studies suggest that, through evolution, nature has developed a way of protecting quantum coherence to enhance the efficiency of photosynthesis. However, critical follow-up studies question the interpretation of these results. Single molecule spectroscopy now shows the quantum characteristics of photosynthesis without the interference of static disorder, and some studies use this method to assign reported signatures of electronic quantum coherence to nuclear dynamics occurring in chromophores.[14][15][16][17][18][19][20] A number of proposals emerged trying to explain unexpectedly long coherence. According to one proposal, if each site within the complex feels its own environmental noise, the electron will not remain in any local minimum due to both quantum coherence and thermal environment, but proceed to the reaction site via quantum walks.[21][22][23] Another proposal is that the rate of quantum coherence and electron tunneling create an energy sink that moves the electron to the reaction site quickly.[24] Other work suggested that geometric symmetries in the complex may favor efficient energy transfer to the reaction center, mirroring perfect state transfer in quantum networks.[25] Furthermore, experiments with artificial dye molecules cast doubts on the interpretation that quantum effects last any longer than one hundred femtoseconds.[26]
In 2017, the first control experiment with the original FMO protein under ambient conditions confirmed that electronic quantum effects are washed out within 60 femtoseconds, while the overall exciton transfer takes a time on the order of a few picoseconds.[27] In 2020 a review based on a wide collection of control experiments and theory concluded that the proposed quantum effects as long lived electronic coherences in the FMO system does not hold.[28] Instead, research investigating transport dynamics suggests that interactions between electronic and vibrational modes of excitation in FMO complexes require a semi-classical, semi-quantum explanation for the transfer of exciton energy. In other words, while quantum coherence dominates in the short-term, a classical description is most accurate to describe long-term behavior of the excitons.[29]
Another process in photosynthesis that has almost 100% efficiency is charge transfer, again suggesting that quantum mechanical phenomena are at play.[20] In 1966, a study on the photosynthetic bacterium Chromatium found that at temperatures below 100 K, cytochrome oxidation is temperature-independent, slow (on the order of milliseconds), and very low in activation energy. The authors, Don DeVault and Britton Chase, postulated that these characteristics of electron transfer are indicative of quantum tunneling, whereby electrons penetrate a potential barrier despite possessing less energy than is classically necessary.[30]
Seth Lloyd is also notable for his contributions to this area of research.
DNA mutation
DNA acts as the instructions for making proteins throughout the body. It consists of 4 nucleotides: guanine, thymine, cytosine, and adenine.[31] The order of these nucleotides gives the “recipe” for the different proteins.
Whenever a cell reproduces, it must copy these strands of DNA. However, sometimes throughout the process of copying the strand of DNA a mutation, or an error in the DNA code, can occur. A theory for the reasoning behind DNA mutation is explained in the Lowdin DNA mutation model.[32] In this model, a nucleotide may spontaneously change its form through a process of quantum tunneling.[33][34] Because of this, the changed nucleotide will lose its ability to pair with its original base pair and consequently changing the structure and order of the DNA strand.
Exposure to ultraviolet lights and other types of radiation can cause DNA mutation and damage. The radiations also can modify the bonds along the DNA strand in the pyrimidines and cause them to bond with themselves creating a dimer.[35]
In many prokaryotes and plants, these bonds are repaired to their original form by a DNA repair enzyme photolyase. As its prefix implies, photolyase is reliant on light in order to repair the strand. Photolyase works with its cofactor FADH, flavin adenine dinucleotide, while repairing the DNA. Photolyase is excited by visible light and transfers an electron to the cofactor FADH-. FADH- now in the possession of an extra electron gives the electron to the dimer to break the bond and repair the DNA. This transfer of the electron is done through the tunneling of the electron from the FADH to the dimer. Although the range of the tunneling is much larger than feasible in a vacuum, the tunneling in this scenario is said to be “superexchange-mediated tunneling,” and is possible due to the protein's ability to boost the tunneling rates of the electron.[32]
Vibration theory of olfaction
Olfaction, the sense of smell, can be broken down into two parts; the reception and detection of a chemical, and how that detection is sent to and processed by the brain. This process of detecting an odorant is still under question. One theory named the “shape theory of olfaction” suggests that certain olfactory receptors are triggered by certain shapes of chemicals and those receptors send a specific message to the brain.[36] Another theory (based on quantum phenomena) suggests that the olfactory receptors detect the vibration of the molecules that reach them and the “smell” is due to different vibrational frequencies, this theory is aptly called the “vibration theory of olfaction.”
The vibration theory of olfaction, created in 1938 by Malcolm Dyson[37] but reinvigorated by Luca Turin in 1996,[38] proposes that the mechanism for the sense of smell is due to G-protein receptors that detect molecular vibrations due to inelastic electron tunneling, tunneling where the electron loses energy, across molecules.[38] In this process a molecule would fill a binding site with a G-protein receptor. After the binding of the chemical to the receptor, the chemical would then act as a bridge allowing for the electron to be transferred through the protein. As the electron transfers across what would otherwise have been a barrier, it loses energy due to the vibration of the newly-bound molecule to the receptor. This results in the ability to smell the molecule.[38][3]
While the vibration theory has some experimental proof of concept,[39][40] there have been multiple controversial results in experiments. In some experiments, animals are able to distinguish smells between molecules of different frequencies and same structure,[41] while other experiments show that people are unaware of distinguishing smells due to distinct molecular frequencies.[42]
Vision
Vision relies on quantized energy in order to convert light signals to an action potential in a process called phototransduction. In phototransduction, a photon interacts with a chromophore in a light receptor. The chromophore absorbs the photon and undergoes photoisomerization. This change in structure induces a change in the structure of the photo receptor and resulting signal transduction pathways lead to a visual signal. However, the photoisomerization reaction occurs at a rapid rate, in under 200 femtoseconds,[43] with high yield. Models suggest the use of quantum effects in shaping the ground state and excited state potentials in order to achieve this efficiency.[44]
Quantum vision implications
Experiments have shown that the sensor in the retina of the human eye is sensitive enough to detect a single photon.[45] Single photon detection could lead to multiple different technologies. One area of development is in quantum communication and cryptography. The idea is to use a biometric system to measure the eye using only a small number of points across the retina with random flashes of photons that “read” the retina and identify the individual.[46] This biometric system would only allow a certain individual with a specific retinal map to decode the message. This message can not be decoded by anyone else unless the eavesdropper were to guess the proper map or could read the retina of the intended recipient of the message.[47]
Enzymatic activity (quantum biochemistry)
Enzymes have been postulated to use quantum tunneling in order to transfer electrons from one place to another in electron transport chains.[48][49][50] It is possible that protein quaternary architectures may have adapted to enable sustained quantum entanglement and coherence, which are two of the limiting factors for quantum tunneling in biological entities.[51] These architectures might account for a greater percentage of quantum energy transfer, which occurs through electron transport and proton tunneling (usually in the form of hydrogen ions, H+).[52][53] Tunneling refers to the ability of a subatomic particle to travel through potential energy barriers.[54] This ability is due, in part, to the principle of complementarity, which holds that certain substances have pairs of properties that cannot be measured separately without changing the outcome of measurement. Particles, such as electrons and protons, have wave-particle duality; they can pass through energy barriers due to their wave characteristics without violating the laws of physics. In order to quantify how quantum tunneling is used in many enzymatic activities, many biophysicists utilize the observation of hydrogen ions. When hydrogen ions are transferred, this is seen as a staple in an organelle's primary energy processing network; in other words, quantum effects are most usually at work in proton distribution sites at distances on the order of an angstrom (1 Å).[55][56] In physics, a semiclassical (SC) approach is most useful in defining this process because of the transfer from quantum elements (e.g. particles) to macroscopic phenomena (e.g. biochemicals). Aside from hydrogen tunneling, studies also show that electron transfer between redox centers through quantum tunneling plays an important role in enzymatic activity of photosynthesis and cellular respiration (see also Mitochondria section below).[57][58] For example, electron tunneling on the order of 15–30 Å contributes to redox reactions in cellular respiration enzymes, such as complexes I, III, and IV in mitochondria.[59][60] Without quantum tunneling, organisms would not be able to convert energy quickly enough to sustain growth.[32] Quantum tunneling actually acts as a shortcut for particle transfer; according to quantum mathematics, a particle's jump from in front of a barrier to the other side of a barrier occurs faster than if the barrier had never been there in the first place. (For more on the technicality of this, see Hartman effect.)
Mitochondria
Organelles, such as mitochondria, are thought to utilize quantum tunneling in order to translate intracellular energy.[61] Traditionally, mitochondria are known to generate most of the cell's energy in the form of chemical ATP. Mitochondria conversion of biomass into chemical ATP is 60-70% efficient, which is superior than the classical regime of man-made engines.[62] To achieve chemical ATP, researchers have found that a preliminary stage before chemical conversion is necessary; this step, via the quantum tunneling of electrons and hydrogen ions (H+), requires a deeper look at the quantum physics that occurs within the organelle.[56]
Because tunneling is a quantum mechanism, it is important to understand how this process may occur for particle transfer in a biological system. Tunneling is largely dependent upon the shape and size of a potential barrier, relative to the incoming energy of a particle.[63] Because the incoming particle can be defined by a wave equation, its tunneling probability is dependent upon the potential barrier's shape in an exponential way, meaning that if the barrier is akin to a very wide chasm, the incoming particle's probability to tunnel will decrease. The potential barrier, in some sense, can come in the form of an actual biomaterial barrier. Mitochondria are encompassed by a membrane structure that is akin to the cellular membrane, on the order of ~75 Å (~7.5 nm) thick.[62] The inner membrane of a mitochondria must be overcome to permit signals (in the form of electrons, protons, H+) to transfer from the site of emittance (internal to the mitochondria) and the site of acceptance (i.e. the electron transport chain proteins).[64] In order to transfer particles, the membrane of the mitochondria must have the correct density of phospholipids to conduct a relevant charge distribution that attracts the particle in question. For instance, for a greater density of phospholipids, the membrane contributes to a greater conductance of protons.[64]
More technically, the form of the mitochondria is the matrix, with inner mitochondrial membranes (IMM) and inner membrane spaces (IMS), all housing protein sites. Mitochondria produce ATP by the oxidation of hydrogen ions from carbohydrates and fats. This process utilizes electrons in an electron transport chain (ETP). The genealogy of electron transport proceeds as follows: Electrons from NADH are transferred to NADH dehydrogenase (complex I protein), which is located in the IMM.[65] Electrons from complex I are transferred to coenzyme Q to make CoQH2; next, electrons flow to cytochrome-containing IMM protein (complex III), which further pushes electrons to cytochrome c, where electrons flow to complex IV; complex IV is the final IMM protein complex of the ETC respiratory chain.[65] This final protein allows electrons to reduce oxygen from an O2 molecule to a single O, so that it can bind to the hydrogen ions to produce H2O. The energy produced from the movement of electrons through the ETC induces proton movement (known as H+ pumping) out of the mitochondria matrix into the IMS.[60] Because any charge movement creates a magnetic field, the IMS now houses a capacitance across the matrix. The capacitance is akin to potential energy, or what is known as a potential barrier. This potential energy guides ATP synthesis via complex V (ATP synthase), which conflates ADP with another P to create ATP by pushing protons (H+) back into the matrix (this process is known as oxidative phosphorylation). Finally, the outer mitochondrial membrane (OMM) houses a voltage-dependent anion channel called the VDAC.[65] This site is important for converting energy signals into electro-chemical outputs for ATP transfer.
Molecular solitons in proteins
Alexander Davydov developed the quantum theory of molecular solitons in order to explain the transport of energy in protein α-helices in general and the physiology of muscle contraction in particular.[66][67] He showed that the molecular solitons are able to preserve their shape through nonlinear interaction of amide I excitons and phonon deformations inside the lattice of hydrogen-bonded peptide groups.[68][69] In 1979, Davydov published his complete textbook on quantum biology entitled "Biology and Quantum Mechanics" featuring quantum dynamics of proteins, cell membranes, bioenergetics, muscle contraction, and electron transport in biomolecules.[9][10]
Magnetoreception
Magnetoreception refers to the ability of animals to navigate using the inclination of the magnetic field of the earth.[70] A possible explanation for magnetoreception is the entangled radical pair mechanism.[71][72] The radical-pair mechanism is well-established in spin chemistry,[73][74][75] and was speculated to apply to magnetoreception in 1978 by Schulten et al.. The ratio between singlet and triplet pairs is changed by the interaction of entangled electron pairs with the magnetic field of the earth.[76] In 2000, cryptochrome was proposed as the "magnetic molecule" that could harbor magnetically sensitive radical-pairs. Cryptochrome, a flavoprotein found in the eyes of European robins and other animal species, is the only protein known to form photoinduced radical-pairs in animals.[70] When it interacts with light particles, cryptochrome goes through a redox reaction, which yields radical pairs both during the photo-reduction and the oxidation. The function of cryptochrome is diverse across species, however, the photoinduction of radical-pairs occurs by exposure to blue light, which excites an electron in a chromophore.[76] Magnetoreception is also possible in the dark, so the mechanism must rely more on the radical pairs generated during light-independent oxidation.
Experiments in the lab support the basic theory that radical-pair electrons can be significantly influenced by very weak magnetic fields, i.e. merely the direction of weak magnetic fields can affect radical-pair's reactivity and therefore can "catalyze" the formation of chemical products. Whether this mechanism applies to magnetoreception and/or quantum biology, that is, whether earth's magnetic field "catalyzes" the formation of biochemical products by the aid of radical-pairs, is undetermined for two reasons. The first is that radical-pairs may need not be entangled, the key quantum feature of the radical-pair mechanism, to play a part in these processes. There are entangled and non-entangled radical-pairs. However, researchers found evidence for the radical-pair mechanism of magnetoreception when European robins, cockroaches, and garden warblers, could no longer navigate when exposed to a radio frequency that obstructs magnetic fields[70] and radical-pair chemistry. To empirically suggest the involvement of entanglement, an experiment would need to be devised that could disturb entangled radical-pairs without disturbing other radical-pairs, or vice versa, which would first need to be demonstrated in a laboratory setting before being applied to in vivo radical-pairs.
Other biological applications
Other examples of quantum phenomena in biological systems include the conversion of chemical energy into motion[77] and brownian motors in many cellular processes.[78]
References
- ↑ Quantum Biology. University of Illinois at Urbana-Champaign, Theoretical and Computational Biophysics Group.
- ↑ Quantum Biology: Powerful Computer Models Reveal Key Biological Mechanism Science Daily Retrieved Oct 14, 2007
- ↑ 3.0 3.1 "Quantum effects in biology: golden rule in enzymes, olfaction, photosynthesis and magnetodetection". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences 473 (2201): 20160822. May 2017. doi:10.1098/rspa.2016.0822. PMID 28588400. Bibcode: 2017RSPSA.47360822B.
- ↑ (in en) How quantum biology might explain life's biggest questions, https://www.ted.com/talks/jim_al_khalili_how_quantum_biology_might_explain_life_s_biggest_questions?language=en, retrieved 2018-12-07
- ↑ "Quantum Biology: Does quantum physics hold the key to revolutionizing medicine?". Progress in Drug Discovery & Biomedical Science 3. 2020. doi:10.36877/pddbs.a0000130.
- ↑ What Is Life?. Berkeley: University of California Press. 1995. p. 1.
- ↑ "Quantum Explorers: Bohr, Jordan, and Delbruck Venturing into Biology". Physics in Perspective 17 (3): 236–250. September 2015. doi:10.1007/s00016-015-0167-7. Bibcode: 2015PhP....17..236J.
- ↑ Lowdin, P.O. (1965) Quantum genetics and the aperiodic solid. Some aspects on the Biological problems of heredity, mutations, aging and tumours in view of the quantum theory of the DNA molecule. Advances in Quantum Chemistry. Volume 2. pp. 213–360. Academic Press
- ↑ 9.0 9.1 Davydov, Alexander S. (1979) (in Russian). Биология и Квантовая Механика [Biology and Quantum Mechanics]. Kiev: Naukova Dumka. OCLC 6736440.
- ↑ 10.0 10.1 Davydov, Alexander S. (1982). Biology and Quantum Mechanics. Oxford: Pergamon Press. ISBN 9780080263922. OCLC 7875407.
- ↑ "Two-dimensional electronic spectroscopy reveals ultrafast energy diffusion in chlorosomes". Journal of the American Chemical Society 134 (28): 11611–11617. July 2012. doi:10.1021/ja3025627. PMID 22690836.
- ↑ "Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems". Nature 446 (7137): 782–786. April 2007. doi:10.1038/nature05678. PMID 17429397. Bibcode: 2007Natur.446..782E. http://ntur.lib.ntu.edu.tw/bitstream/246246/221945/-1/04.pdf.
- ↑ "Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature". Nature 463 (7281): 644–647. February 2010. doi:10.1038/nature08811. PMID 20130647. Bibcode: 2010Natur.463..644C.
- ↑ "Vibrational beatings conceal evidence of electronic coherence in the FMO light-harvesting complex". The Journal of Physical Chemistry B 118 (45): 12865–12872. November 2014. doi:10.1021/jp510074q. PMID 25321492.
- ↑ "Origin of long-lived coherences in light-harvesting complexes". The Journal of Physical Chemistry B 116 (25): 7449–7454. June 2012. doi:10.1021/jp304649c. PMID 22642682. Bibcode: 2012arXiv1201.6325C.
- ↑ "Vibrational vs. electronic coherences in 2D spectrum of molecular systems". Chem. Phys. Lett. 545 (30): 40–43. 2012. doi:10.1016/j.cplett.2012.07.014. Bibcode: 2012CPL...545...40B.
- ↑ "Electronic resonance with anticorrelated pigment vibrations drives photosynthetic energy transfer outside the adiabatic framework". Proceedings of the National Academy of Sciences of the United States of America 110 (4): 1203–1208. January 2013. doi:10.1073/pnas.1211157110. PMID 23267114.
- ↑ "Exciton Structure and Energy Transfer in the Fenna-Matthews-Olson Complex". The Journal of Physical Chemistry Letters 7 (9): 1653–1660. May 2016. doi:10.1021/acs.jpclett.6b00534. PMID 27082631. https://lup.lub.lu.se/record/b1c8070b-60cf-4e41-8895-ea13faf95777.
- ↑ "Impact of environmentally induced fluctuations on quantum mechanically mixed electronic and vibrational pigment states in photosynthetic energy transfer and 2D electronic spectra". The Journal of Chemical Physics 142 (21): 212403. June 2015. doi:10.1063/1.4914302. PMID 26049423. Bibcode: 2015JChPh.142u2403F.
- ↑ 20.0 20.1 "The future of quantum biology". Journal of the Royal Society, Interface 15 (148): 20180640. November 2018. doi:10.1098/rsif.2018.0640. PMID 30429265.
- ↑ "Environment-assisted quantum walks in photosynthetic energy transfer". The Journal of Chemical Physics 129 (17): 174106. November 2008. doi:10.1063/1.3002335. PMID 19045332. Bibcode: 2008JChPh.129q4106M.
- ↑ "Dephasing-assisted transport: quantum networks and biomolecules – IOPscience". New Journal of Physics 10 (11): 113019. 2008-11-01. doi:10.1088/1367-2630/10/11/113019. Bibcode: 2008NJPh...10k3019P.
- ↑ (Speech). From Atomic to Mesoscale: The Role of Quantum Coherence in Systems of Various Complexities. Institute for Theoretical, Atomic and Molecular and Optical Physics, Harvard-Smithsonian Center for Astrophysics, Cambridge, Massachusetts. 2014-03-10 https://www.youtube.com/watch?v=YC28RzLeuFc. Retrieved 2019-09-30. Missing or empty
|title=(help) - ↑ "Quantum coherence accelerating photosynthetic energy transfer". Ultrafast Phenomena XVI. Springer Series in Chemical Physics. 92. 2009. 607–609. doi:10.1007/978-3-540-95946-5_197. ISBN 978-3-540-95945-8. Bibcode: 2009up16.book..607L. http://chemport.cas.org/cgi-bin/sdcgi?APP=ftslink&action=reflink&origin=npg&version=1.0&coi=1:CAS:528:DC%2BC3cXnvVyru70%3D&pissn=1745-2473&pyear=2012&md5=c5c038282de4853fa562a585bdcd6868.
- ↑ "Optimally designed quantum transport across disordered networks". Physical Review Letters 111 (18): 180601. November 2013. doi:10.1103/PhysRevLett.111.180601. PMID 24237498. Bibcode: 2013PhRvL.111r0601W.
- ↑ "Two-dimensional spectroscopy of a molecular dimer unveils the effects of vibronic coupling on exciton coherences". Nature Chemistry 6 (3): 196–201. March 2014. doi:10.1038/nchem.1834. PMID 24557133. Bibcode: 2014NatCh...6..196H.
- ↑ "Nature does not rely on long-lived electronic quantum coherence for photosynthetic energy transfer". Proceedings of the National Academy of Sciences of the United States of America 114 (32): 8493–8498. August 2017. doi:10.1073/pnas.1702261114. PMID 28743751. Bibcode: 2017PNAS..114.8493D.
- ↑ "Quantum biology revisited". Science Advances 6 (14): eaaz4888. April 2020. doi:10.1126/sciadv.aaz4888. PMID 32284982. Bibcode: 2020SciA....6.4888C.
- ↑ "Vibrations, quanta and biology". Contemporary Physics 54 (4): 181–207. 2013-07-01. doi:10.1080/00405000.2013.829687. ISSN 0010-7514. Bibcode: 2013ConPh..54..181H.
- ↑ "Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling". Biophysical Journal 6 (6): 825–847. November 1966. doi:10.1016/S0006-3495(66)86698-5. PMID 5972381. Bibcode: 1966BpJ.....6..825D.
- ↑ "DNA and Mutations". https://evolution.berkeley.edu/evolibrary/article/mutations_01.
- ↑ 32.0 32.1 32.2 "Quantum Tunnelling to the Origin and Evolution of Life". Current Organic Chemistry 17 (16): 1758–1770. August 2013. doi:10.2174/13852728113179990083. PMID 24039543.
- ↑ "Quantum and classical effects in DNA point mutations: Watson-Crick tautomerism in AT and GC base pairs". Physical Chemistry Chemical Physics 23 (7): 4141–4150. February 2021. doi:10.1039/D0CP05781A. PMID 33533770. Bibcode: 2021PCCP...23.4141S.
- ↑ Slocombe, Louie; Sacchi, Marco; Al-Khalili, Jim (2022-05-05). "An open quantum systems approach to proton tunnelling in DNA" (in en). Communications Physics 5 (1): 1–9. doi:10.1038/s42005-022-00881-8. ISSN 2399-3650. https://www.nature.com/articles/s42005-022-00881-8.
- ↑ "Ultraviolet radiation: DNA damage, repair, and human disorders" (in en). Molecular & Cellular Toxicology 13 (1): 21–28. March 2017. doi:10.1007/s13273-017-0002-0. ISSN 1738-642X.
- ↑ "Olfactory theories and the odors of small molecules". Journal of Agricultural and Food Chemistry 19 (5): 999–1004. May 1971. doi:10.1021/jf60177a002. PMID 5134656.
- ↑ "The scientific basis of odour" (in en). Journal of the Society of Chemical Industry 57 (28): 647–651. 1938-07-09. doi:10.1002/jctb.5000572802. ISSN 0368-4075.
- ↑ 38.0 38.1 38.2 "A spectroscopic mechanism for primary olfactory reception". Chemical Senses 21 (6): 773–791. December 1996. doi:10.1093/chemse/21.6.773. PMID 8985605.
- ↑ "Odorant shape and vibration likely lead to olfaction satisfaction". https://phys.org/news/2012-09-odorant-vibration-olfaction-satisfaction.html.
- ↑ "A Novel Multigene Family May Encode Odorant Receptors: A Molecular Basis for Odor Recognition". April 5, 1991. http://www.percepnet.com/documenta/Cell_1991.pdf.
- ↑ "The role of metals in mammalian olfaction of low molecular weight organosulfur compounds". Natural Product Reports 34 (5): 529–557. May 2017. doi:10.1039/c7np00016b. PMID 28471462.
- ↑ "A psychophysical test of the vibration theory of olfaction" (in En). Nature Neuroscience 7 (4): 337–338. April 2004. doi:10.1038/nn1215. PMID 15034588.
- ↑ "The Primary Photochemistry of Vision Occurs at the Molecular Speed Limit". The Journal of Physical Chemistry B 121 (16): 4040–4047. April 2017. doi:10.1021/acs.jpcb.7b02329. PMID 28358485. https://pure.rug.nl/ws/files/42527095/acs_2Ejpcb_2E7b02329.pdf.
- ↑ "The first step in vision: femtosecond isomerization of rhodopsin". Science 254 (5030): 412–415. October 1991. doi:10.1126/science.1925597. PMID 1925597. Bibcode: 1991Sci...254..412S.
- ↑ "The Human Eye and Single Photons". http://math.ucr.edu/home/baez/physics/Quantum/see_a_photon.html.
- ↑ "Quantum Biometrics with Retinal Photon Counting". Physical Review Applied 8 (4): 044012. 2017. doi:10.1103/PhysRevApplied.8.044012. Bibcode: 2017PhRvP...8d4012L.
- ↑ Emerging Technology from the arXiv. "The unique way your eyes detect photons could be used to guarantee your identity, say physicists" (in en). MIT Technology Review. https://www.technologyreview.com/s/604266/quantum-biometrics-exploits-the-human-eyes-ability-to-detect-single-photons/.
- ↑ "On the Theory of Oxidation‐Reduction Reactions Involving Electron Transfer. I" (in en). The Journal of Chemical Physics 24 (5): 966–978. May 1956. doi:10.1063/1.1742723. ISSN 0021-9606. Bibcode: 1956JChPh..24..966M. http://aip.scitation.org/doi/10.1063/1.1742723.
- ↑ "Editorial". Photosynthesis Research 22 (1): 1. January 1989. doi:10.1007/BF00114760. PMID 24424672.
- ↑ "Electron tunneling through proteins". Quarterly Reviews of Biophysics 36 (3): 341–372. August 2003. doi:10.1017/S0033583503003913. PMID 15029828.
- ↑ Apte SP, Quantum biology: Harnessing nano-technology’s last frontier with modified excipients and food ingredients, J. Excipients and Food Chemicals, 5(4), 177–183, 2014
- ↑ "Extremely Large Isotope Effects in the Soybean Lipoxygenase-Linoleic Acid Reaction" (in en). Journal of the American Chemical Society 116 (2): 793–794. January 1994. doi:10.1021/ja00081a060. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00081a060.
- ↑ "Tunneling and dynamics in enzymatic hydride transfer". Chemical Reviews 106 (8): 3095–3118. August 2006. doi:10.1002/chin.200643274. PMID 16895320.
- ↑ Introduction to quantum mechanics (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. 2005. ISBN 0-13-111892-7. OCLC 53926857. https://www.worldcat.org/oclc/53926857.
- ↑ "Atomic description of an enzyme reaction dominated by proton tunneling". Science 312 (5771): 237–241. April 2006. doi:10.1126/science.1126002. PMID 16614214. Bibcode: 2006Sci...312..237M.
- ↑ 56.0 56.1 Physical biology : from atoms to medicine. Ahmed H. Zewail. London, UK: Imperial College Press. 2008. ISBN 978-1-84816-201-3. OCLC 294759396. https://www.worldcat.org/oclc/294759396.
- ↑ "Electron tunneling through proteins". Quarterly Reviews of Biophysics 36 (3): 341–372. August 2003. doi:10.1017/S0033583503003913. PMID 15029828.
- ↑ "Tunneling and dynamics in enzymatic hydride transfer". Chemical Reviews 106 (8): 3095–3118. August 2006. doi:10.1021/cr050301x. PMID 16895320.
- ↑ "Quantum biology". Nature Physics 9 (1): 10–18. 2013-01-01. doi:10.1038/nphys2474. ISSN 1745-2473. Bibcode: 2013NatPh...9...10L.
- ↑ 60.0 60.1 "Adrenal Mitochondria and Steroidogenesis: From Individual Proteins to Functional Protein Assemblies". Frontiers in Endocrinology 7: 106. 2016-07-29. doi:10.3389/fendo.2016.00106. PMID 27524977.
- ↑ "The quantum mitochondrion and optimal health". Biochemical Society Transactions 44 (4): 1101–1110. August 2016. doi:10.1042/BST20160096. PMID 27528758.
- ↑ 62.0 62.1 Energy Flow in Biology. New York and London: Academic Press. 1968. pp. 55–56; 103–105; 116.
- ↑ "Quantum physics meets biology". HFSP Journal 3 (6): 386–400. December 2009. doi:10.2976/1.3244985. PMID 20234806.
- ↑ 64.0 64.1 "A quantum origin of life?". Quantum aspects of life. Imperial College Press. January 2008. pp. 3–18. doi:10.1142/9781848162556_0001. ISBN 978-1-84816-253-2.
- ↑ 65.0 65.1 65.2 "Mitochondrial form and function". Nature 505 (7483): 335–343. January 2014. doi:10.1038/nature12985. PMID 24429632. Bibcode: 2014Natur.505..335F.
- ↑ Davydov, Alexander S. (1973). "The theory of contraction of proteins under their excitation". Journal of Theoretical Biology 38 (3): 559–569. doi:10.1016/0022-5193(73)90256-7. PMID 4266326. Bibcode: 1973JThBi..38..559D.
- ↑ Davydov, Alexander S. (1977). "Solitons and energy transfer along protein molecules". Journal of Theoretical Biology 66 (2): 379–387. doi:10.1016/0022-5193(77)90178-3. PMID 886872. Bibcode: 1977JThBi..66..379D.
- ↑ Davydov, Alexander S. (1982). "Solitons in quasi-one-dimensional molecular structures". Soviet Physics Uspekhi 25 (12): 898–918. doi:10.1070/pu1982v025n12abeh005012.
- ↑ Scott, Alwyn C. (1985). "Davydov solitons in polypeptides". Philosophical Transactions of the Royal Society of London Series A, Mathematical and Physical Sciences 315 (1533): 423–436. doi:10.1098/rsta.1985.0049. Bibcode: 1985RSPTA.315..423S. https://digital.library.unt.edu/ark:/67531/metadc1210664/.
- ↑ 70.0 70.1 70.2 "The Radical-Pair Mechanism of Magnetoreception". Annual Review of Biophysics 45 (1): 299–344. July 2016. doi:10.1146/annurev-biophys-032116-094545. PMID 27216936. https://ora.ox.ac.uk/objects/uuid:c1e3c8ca-98b3-4e9d-8efd-0b9ad9b965eb.
- ↑ "A Biomagnetic Sensory Mechanism Based on Magnetic Field Modulated Coherent Electron Spin Motion : Zeitschrift für Physikalische Chemie". Zeitschrift für Physikalische Chemie 111: 1–5. 1978. doi:10.1524/zpch.1978.111.1.001.
- ↑ "The radical-pair mechanism as a paradigm for the emerging science of quantum biology". Mod. Phys. Lett. B 29: 1530013. 2015. doi:10.1142/S0217984915300136. Bibcode: 2015MPLB...29S0013K.
- ↑ "Magnetic field effects in chemical systems". Pure and Applied Chemistry 81 (1): 19–43. 2009-01-01. doi:10.1351/PAC-CON-08-10-18. ISSN 1365-3075.
- ↑ "Magnetic field effects in chemical kinetics and related phenomena". Chemical Reviews 89 (1): 51–147. 1989-01-01. doi:10.1021/cr00091a003. ISSN 0009-2665. http://nbn-resolving.de/urn:nbn:de:bsz:352-opus-46797.
- ↑ "Radical Pairs in Solution". Progress in Reaction Kinetics and Mechanism 27 (3): 165–207. 2002-09-01. doi:10.3184/007967402103165388.
- ↑ 76.0 76.1 "Light-dependent magnetoreception in birds: the crucial step occurs in the dark". Journal of the Royal Society, Interface 13 (118): 20151010. May 2016. doi:10.1098/rsif.2015.1010. PMID 27146685.
- ↑ Molecular Reaction Dynamics. Cambridge University Press. 2005. pp. 16–18. ISBN 978-0-521-84276-1. https://archive.org/details/molecularreactio00levi_0/page/16.
- ↑ Nanotechnology: Assessment and Perspectives. Springer-Verlag Berlin and Heidelberg GmbH & Co. K. 2006. pp. 197–240. ISBN 978-3-540-32819-3.
External links
- Philip Ball (2015). "Quantum Biology: An Introduction". The Royal Institution
- Quantum Biology and the Hidden Nature of Nature, World Science Festival 2012, video of podium discussion
- Quantum Biology: Current Status and Opportunities, September 17-18, 2012, University of Surrey, UK
 |