Biology:Chaetognatha
The Chaetognatha /kiːˈtɒɡnəθə/ or chaetognaths /ˈkiːtɒɡnæθs/ (meaning bristle-jaws) are a phylum of predatory marine worms that are a major component of plankton worldwide. Commonly known as arrow worms, they are mostly pelagic; however about 20% of the known species are benthic, and can attach to algae and rocks. They are found in all marine waters, from surface tropical waters and shallow tide pools to the deep sea and polar regions. Most chaetognaths are transparent and are torpedo shaped, but some deep-sea species are orange. They range in size from 2 to 120 millimetres (0.1 to 4.7 in).
Chaetognaths were first recorded by the Dutch naturalist Martinus Slabber in 1775.[1] As of 2021, biologists recognize 133 modern species assigned to over 26 genera and eight families.[1] Despite the limited diversity of species, the number of individuals is large.[2]
Arrow worms are strictly related to and possibly belonging to Gnathifera, a clade of protostomes that do not belong to either Ecdysozoa or Lophotrochozoa.
Anatomy

Chaetognaths are transparent or translucent dart-shaped animals covered by a cuticle. They range in length between 1.5 mm to 105 mm in the Antarctic species Pseudosagitta gazellae.[3] Body size, either between individuals in the same species or between different species, seems to increase with decreasing temperature.[3] The body is divided into a distinct head, trunk, and tail. About 80% of the body is occupied by primary longitudinal muscles.[1]
Head and digestive system
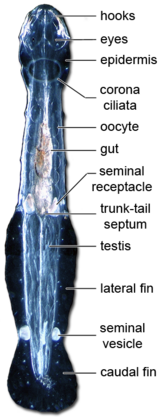
There are between four and fourteen hooked, grasping spines on each side of their head, flanking a hollow vestibule containing the mouth. The spines are used in hunting, and covered with a flexible hood arising from the neck region when the animal is swimming. Spines and teeth are made of α-chitin, and the head is protected by a chitinous armature.[1]

The mouth opens into a muscular pharynx, which contains glands to lubricate the passage of food. From here, a straight intestine runs the length of the trunk to an anus just forward of the tail. The intestine is the primary site of digestion and includes a pair of diverticula near the anterior end.[4] Materials are moved about the body cavity by cilia. Waste materials are simply excreted through the skin and anus. Eukrohniid species possess an oil vacuole closely associated with the gut. This organ contains wax esters which may assist reproduction and growth outside of the production season for Eukrohnia hamata in Arctic seas.[5] Owing to the position of the oil vacuole in the center of the tractus, the organ may also have implications for buoyancy, trim and locomotion.[6]
Usually chaetognaths are not pigmented, however the intestines of some deep-sea species contain orange-red carotenoid pigments.[1]
Nervous and sensory systems
The nervous system is reasonably simple and shows a typical protostome anatomy,[1] consisting of a ganglionated nerve ring surrounding the pharynx. The brain is composed of two distinct functional domains: the anterior neuropil domain and the posterior neuropil domain. The former probably controls head muscles moving the spines and the digestive system. The latter is linked to eyes and the corona ciliata. A putative sensory structure of unknown function, the retrocerebral organ, is also hosted by the posterior neuropil domain.[1] The ventral ganglion is the largest, but nerves extend from all the ganglia along the length of the body.
Chaetognaths have two compound eyes, each consisting of a number of pigment-cup ocelli fused together; some deep-sea and troglobitic species have unpigmented or absent eyes.[1] In addition, there are a number of sensory bristles arranged in rows along the side of the body, where they probably perform a function similar to that of the lateral line in fish. An additional, curved, band of sensory bristles lies over the head and neck.[4] Almost all chaetognaths have "indirect" or "inverted" eyes, according to the orientation of photoreceptor cells; only some Eukhroniidae species have "direct" or "everted" eyes.[1] A unique feature of the chaetognath eye is the lamellar structure of photoreceptor membranes, containing a grid of 35–55 nm wide circular pores.[1]
A significant mechanosensory system, composed of ciliary receptor organs, detects vibrations, allowing chaetognaths to detect the swimming motion of potential prey. Another organ on the dorsal part of the neck, the corona ciliata, is probably involved in chemoreception.[1]
Internal organs
The body cavity is lined by peritoneum, and therefore represents a true coelom, and is divided into one compartment on each side of the trunk, and additional compartments inside the head and tail, all separated completely by septa. Although they have a mouth with one or two rows of tiny teeth, compound eyes, and a nervous system, they have no excretory or respiratory systems.[7][1] While often said to lack a circulatory system, chaetognaths do have a rudimentary hemal system resembling those of annelids.[1]
The arrow worm rhabdomeres are derived from microtubules 20 nm long and 50 nm wide, which in turn form conical bodies that contain granules and thread structures. The cone body is derived from a cilium.[8]
Locomotion
The trunk bears one or two pairs of lateral fins incorporating structures superficially similar to the fin rays of fish, with which they are not homologous. Unlike those of vertebrates, these lateral fins are composed of a thickened basement membrane extending from the epidermis. An additional caudal fin covers the post-anal tail.[4] Two chaetognath species, Caecosagitta macrocephala and Eukrohnia fowleri, have bioluminescent organs on their fins.[9][10]
Chaetognaths swim in short bursts using a dorso-ventral undulating body motion, where their tail fin assists with propulsion and the body fins with stabilization and steering.[11] Muscle movements have been described as among the fastest in metazoans.[1] Muscles are directly excitable by electrical currents or strong K+ solutions; the main neuromuscular transmitter is acetylcholine.[1]
Reproduction and life cycle
All species are hermaphroditic, carrying both eggs and sperm.[2] Each animal possesses a pair of testes within the tail, and a pair of ovaries in the posterior region of the main body cavity. Immature sperm are released from the testes to mature inside the cavity of the tail, and then swim through a short duct to a seminal vesicle where they are packaged into a spermatophore.[4]
During mating, each individual places a spermatophore onto the neck of its partner after rupture of the seminal vesicle. The sperm rapidly escape from the spermatophore and swim along the midline of the animal until they reach a pair of small pores just in front of the tail. These pores connect to the oviducts, into which the developed eggs have already passed from the ovaries, and it is here that fertilisation takes place.[4] The seminal receptacles and oviducts accumulate and store spermatozoa, to perform multiple fertilisation cycles.[1] Some benthic members of Spadellidae are known to have elaborate courtship rituals before copulation,[1] for example Paraspadella gotoi.[12]
The eggs are mostly planktonic, except in a few species such as Ferosagitta hispida that attaches eggs to the substrate.[1] In Eukrohnia, eggs develop in marsupial sacs or attached to algae.[13] Eggs usually hatch after 1–3 days. Chaetognaths do not undergo metamorphosis nor they possess a well-defined larval stage,[4][1] an unusual trait among marine invertebrates;[12] however there are significant morphological differences between the newborn and the adult, with respect to proportions, chitinous structures and fin development.[1][14]
The life spans of chaetognaths are variable but short; the longest recorded was 15 months in Sagitta friderici.[14]
Behaviour
Little is known of arrow worms' behaviour and physiology, due to the complexity in culturing them and reconstructing their natural habitat.[1] It is known that they feed more frequently with higher temperatures. Planktonic chaetognaths often must swim continuously, with a "hop and sink" behaviour, to keep themselves in the desired location in the water layer, and swim actively to catch prey. They all tend to keep the body slightly slanted with the head pointing downwards.[1] They often show a "gliding" behaviour, slowly sinking for a while, and then catching up with a quick movement of their fins.[12] Benthic species usually stay attached to substrates such as rocks, algae or sea grasses, more rarely on top or between sand grains, and act more strictly as ambush predators, staying still until prey passes by.[1] The prey is detected thanks to the ciliary fence and tuft organs, sensing vibrations[1] – individuals of Spadella cephaloptera for example will attack a glass or metal probe vibrating at an adequate frequency.[12] To catch prey, arrow worms jump forward with a strong stroke of the tail fin.[1] Once in contact with prey, they withdraw the hood over the grasping spines, so that it forms a cage around the prey and bring it in contact with the mouth. They swallow their prey whole.[12]
Ecology
Chaetognaths are found in all world's oceans, from the poles to tropics, and also in brackish and estuarine waters. They inhabit very diverse environments, from hydrothermal vents to deep ocean seafloor, to seagrass beds and marine caves.[1] The majority are planktonic, and they are often the second most common component of zooplankton, with a biomass ranging between 10 and 30% that of copepods.[1] In the Canada Basin, chaetognaths alone represent ~13% of the zooplankton biomass.[15] As such, they are ecologically relevant and a key food source for fishes and other predators, including commercially relevant fishes such as mackerel or sardines.[16] 58% of known species are pelagic,[3] while about a third of species are epibenthic or meiobenthic, or inhabit the immediate vicinity of the substrate.[1] Chaetognaths have been recorded up to 5000 and possibly even 6000 meters of depth.[3]
The highest density of chaetognaths is observed in the photic zone of shallow waters.[1] Larger chaetognath species tend to live deeper in water, but spend their juvenile stages higher in the water column.[12] Arrow worms however engage in diel vertical migration, spending the day at lower depths to avoid predators, and coming close to the surface at night. Their position in the water column can depend on light, temperature, salinity, age and food supply. They cannot swim against oceanic currents, and they are used as a hydrological indicator of currents and water masses.[1]
All chaetognaths are ambush predators, preying on other planktonic animals, mostly copepods and cladocerans[4][1] but also amphipods, krill and fish larvae.[16] Adults can feed on younger individuals of the same species.[17] Some species are also reported to be omnivores, feeding on algae and detritus.[18] Chaetognaths are known to use the neurotoxin tetrodotoxin to subdue prey,[19] possibly synthesized by Vibrio bacterial species.[1]
Genetics
In 2025, the genome of the arrow worm Paraspadella gotoi has been published. The genome is 257 Mb long, consists of 9 chromosomes and includes 22.072 protein-coding genes, with 20% of the sequence being occupied by repeats.[20] The genome lacks genes for the centromeric histone H3 and CENPT, both proteins involved in the centromere; a condition associated with faster rearrangements of the genome in other species. Accordingly, genomic analysis indicates a higher rate of chromosomal rearrangement in gnathiferans. The P.gotoi genome also shows evidence of a significant gene duplication event, probably due to a burst of tandem gene duplication (a rare condition in animal genomes) instead than to a whole genome duplication event, involving 3.379 gene families. Many of these genes are involved in development and ion transmembrane transport.[20] Chaetognaths seem to include as many as 2.250 unique gene families, a large number compared with the 157 unique gene families of Mollusca or 124 unique gene families of Echinodermata.[20] Nearly half the chaetognath genes undergo trans-splicing.[20]
Mitochondrial genome
The mtDNA of the arrow worm Spadella cephaloptera has been sequenced in 2004, and at the time it was the smallest metazoan mitochondrial genome known, being 11,905 base pairs long[21] (it has now been surpassed by the mitchondrial genome of the ctenophore Mnemiopsis leidyi, which is 10,326 bp long).[22] All mitochondrial tRNA genes are absent. The MT-ATP8 and MT-ATP6 genes are also missing.[21] The mtDNA of Paraspadella gotoi, also sequenced in 2004, is even smaller (11,403 bp) and it shows a similar pattern, lacking 21 of the 22 usually present tRNA genes and featuring only 14 of the 37 genes normally present.[23]
Chaetognaths show a unique mitochondrial genomic diversity within individual of the same species.[24]
Phylogeny and evolution

Evolution and relationship to other animals
The evolutionary relationships of chaetognaths have long been enigmatic. Charles Darwin remarked that arrow worms were "remarkable for the obscurity of their affinities".[12] Chaetognaths in the past have been traditionally, but erroneously, classed as deuterostomes by embryologists due to deuterostome-like features in the embryo. Lynn Margulis and K. V. Schwartz placed chaetognaths in the deuterostomes in their Five Kingdom classification.[26] However, several developmental features are at odds with deuterostomes and are either akin to Spiralia or unique to Chaetognatha.[1]
| ||||||||||||||||||||||||||||||||||||
| Summary of relationships of gnathiferans in recent studies including Chaetognatha within the clade, with disputed relationships represented as polytomies[27][28][29][30][31] |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chaetognaths in the metazoan tree of life, when considered the sister group of Gnathifera.[1] |
Molecular phylogeny shows that Chaetognatha are, in fact, protostomes. Thomas Cavalier-Smith places them in the protostomes in his Six Kingdom classification.[32] The similarities between chaetognaths and nematodes mentioned above may support the protostome thesis—in fact, chaetognaths are sometimes regarded as a basal ecdysozoan or lophotrochozoan.[33] Chaetognatha appears close to the base of the protostome tree in most studies of their molecular phylogeny.[34] This may explain their deuterostome embryonic characters. If chaetognaths branched off from the protostomes before they evolved their distinctive protostome embryonic characters, they might have retained deuterostome characters inherited from early bilaterian ancestors. Thus chaetognaths may be a useful model for the ancestral bilaterian.[35] Studies of arrow worms' nervous systems suggests they should be placed within the protostomes.[36][37] According to 2017 and 2019 papers, chaetognaths either belong to[38][39] or are the sister group of Gnathifera.[1]
In 2025, the sequencing of Paraspadella gotoi revealed that chaetognaths underwent both extensive gene loss and gene duplication and feature an unusual number of phylum-specific gene families that seem to have an important role in shaping novel cell types. One of the duplication events involved the Hox cluster, probably playing a role in sculpting the unique chaetognath body plan.[20] Overall, according to researchers, the chaetognath body plan seems to have evolved not by incorporating and adapting the ancestral bilaterian genetic toolkit, but by discarding most of it and then evolving its own new genes, thus explaining at least in part the enigmatic uniqueness of these animals.[20]
Fossil record
Due to their soft bodies, chaetognaths fossilize poorly. Even so, several fossil chaetognath species have been described.[40] Chaetognaths first appear during the Cambrian Period. Complete body fossils have been formally described from the Lower Cambrian Maotianshan shales of Yunnan, China (Eognathacantha ercainella Chen & Huang[41] and Protosagitta spinosa Hu[42]) and the Middle Cambrian Burgess Shale of British Columbia (Capinatator praetermissus.) A Cambrian stem-group chaetognath, Timorebestia, first described in 2024, was much larger than modern species, showing that chaetognaths occupied different roles in marine ecosystems compared to today.[43] A more recent chaetognath, Paucijaculum samamithion Schram, has been described from the Mazon Creek biota from the Pennsylvanian of Illinois. The enigmatic Nectocaris, documented from the Burgess Shale since 1910 and described in 1976, has been recognized as a stem-chateognath in 2025.[44]
Chaetognaths were thought possibly to be related to some of the animals grouped with the conodonts. The conodonts themselves, however, have been shown to be dental elements of vertebrates. It is now thought that protoconodont elements (e.g., Protohertzina anabarica Missarzhevsky, 1973), are probably grasping spines of chaetognaths rather than teeth of conodonts. Previously chaetognaths in the Early Cambrian were only suspected from these protoconodont elements, but the more recent discoveries of body fossils have confirmed their presence then.[45] There is evidence that chaetognaths were important components of the oceanic food web already in the Early Cambrian.[46]
Internal phylogeny
Below is a consensus evolutionary tree of extant Chaetognatha, based on both morphological and molecular data, as of 2021.[1]
| Chaetognatha |
| ||||||||||||||||||||||||
History
The first known description of a chaetognath has been published by Dutch naturalist Martinus Slabber in the 1770s; he also coined the name "arrow worm".[47][3] The zoologist Henri Marie Ducrotay de Blainville also briefly mentioned probable chaetognaths but he understood them as pelagic mollusks. The first description of a currently accepted species of chaetognath, Sagitta bipunctata, is from 1827.[48][3] Among the early zoologists describing arrow worms, there is Charles Darwin, who took notes about them during the voyage of the Beagle and in 1844 dedicated a paper to them.[49] In the following year, August David Krohn published an early anatomical description of Sagitta bipunctata.[50][16]
The term "chaetognath" has been coined in 1856 by Rudolf Leuckart. He was also the first to propose that the genus Sagitta belonged to a separate group: «At the moment, it seems most natural to regard the Sagittas as representatives of a small group of their own that makes the transition from the real annelids (first of all the lumbricines) to the nematodes, and may not be unsuitably named Chaetognathi.»[51][3]
The modern systematics of Chaetognatha begins in 1911 with Ritter-Záhony[52][16] and is later consolidated by Takasi Tokioka in 1965[53][3][16] and Robert Bieri in 1991.[54] Tokioka introduced the orders Phragmophora and Aphragmophora, and classified four families, six genera, for a total of 58 species – plus the extinct Amiskwia, classified as a true primitive chaetognath in a separate class, Archisagittoidea.[16]
Chaetognaths were for a while considered as belonging or affine to the deuterostomes, but suspects of their affinities among Spiralia or other protostomes were already present as early as 1986.[21] Their affinities with protostomes were clarified in 2004 by sequencing and analysis of mtDNA.[21]
Infection by giant viruses
File:Comparison of the size of giant viruses to a common virus (HIV) and bacteria (E. coli).tif In 2018, reanalysis of electron microscopy photographs from the 1980s allowed scientists to identify a giant virus (Meelsvirus) infecting Adhesisagitta hispida; its site of multiplication is nuclear and the virions (length: 1.25 μm) are enveloped.[55] In 2019, reanalysis of other previous studies has shown that structures that were taken in 1967 for bristles present on the surface of the species Spadella cephaloptera,[56] and in 2003, for bacteria infecting Paraspadella gotoi,[57] were in fact enveloped and spindle-shaped giant viruses with a cytoplasmic site of multiplication.[58] The viral species infecting P. gotoi, whose maximum length is 3.1 μm, has been named Klothovirus casanovai (Klotho being the Greek name for one of the three Fates whose attribute was a spindle, and casanovai, in tribute to Pr J.-P. Casanova who devoted a large part of his scientific life to the study of chaetognaths). The other species has been named Megaklothovirus horridgei (in tribute to Adrian Horridge, the first author of the 1967 article). On a photograph, one of the viruses M. horridgei, although truncated, is 3.9 μm long, corresponding to about twice the length of the bacteria Escherichia coli. Many ribosomes are present in virions but their origin remains unknown (cellular, viral or only partly viral). To date, giant viruses known to infect metazoans are exceptionally rare.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 Perez, Yvan; Müller, Carsten H.G.; Harzsch, Steffen (2021). "Chapter 15: Chaetognatha". in Schierwater, Bernd. Invertebrate Zoology: A Tree of Life Approach. CRC Press. p. 231. ISBN 978-1-4822-3582-1. https://books.google.com/books?id=Bk4vEAAAQBAJ&pg=PA231. Retrieved 2023-08-14.
- ↑ 2.0 2.1 The Biology of Chaetognaths. London: Oxford University Press. 1991. ISBN 978-0-19-857715-7.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Pauly, Daniel; Liang, Cui; Xian, Weiwei; Chu, Elaine; Bailly, Nicolas (2021). "The Sizes, Growth and Reproduction of Arrow Worms (Chaetognatha) in Light of the Gill-Oxygen Limitation Theory (GOLT)". Journal of Marine Science and Engineering 9 (12): 1397. doi:10.3390/jmse9121397.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 1046–1050. ISBN 978-0-03-056747-6.
- ↑ Grigor, Jordan J.; Schmid, Moritz S.; Fortier, Louis (2017-11-01). "Growth and reproduction of the chaetognaths Eukrohnia hamata and Parasagitta elegans in the Canadian Arctic Ocean: capital breeding versus income breeding" (in en). Journal of Plankton Research 39 (6): 910–929. doi:10.1093/plankt/fbx045. ISSN 0142-7873. https://academic.oup.com/plankt/article/39/6/910/4344873.
- ↑ Pond, David W. (2012-06-01). "The physical properties of lipids and their role in controlling the distribution of zooplankton in the oceans" (in en). Journal of Plankton Research 34 (6): 443–453. doi:10.1093/plankt/fbs027. ISSN 0142-7873. https://academic.oup.com/plankt/article/34/6/443/1576677.
- ↑ Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth
- ↑ "Photoreception". Encyclopædia Britannica from Encyclopædia Britannica 2006 Ultimate Reference Suite DVD . 2009.
- ↑ "A bioluminescent chaetognath". Nature 367 (6460): 225–226. 20 January 1994. doi:10.1038/367225a0. Bibcode: 1994Natur.367..225H.
- ↑ "Bioluminescent organs of two deep-sea arrow worms, Eukrohnia fowleri and Caecosagitta macrocephala, with further observations on Bioluminescence in chaetognaths". The Biological Bulletin 219 (2): 100–11. October 2010. doi:10.1086/BBLv219n2p100. PMID 20972255.
- ↑ "A model of rapid-start swimming at intermediate Reynolds number: undulatory locomotion in the chaetognath Sagitta elegans". Journal of Experimental Biology 163 (1): 119–137. 1992. doi:10.1242/jeb.163.1.119. http://jeb.biologists.org/content/163/1/119.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Ball, Eldon E.; Miller, David J. (2006). "Phylogeny: The Continuing Classificatory Conundrum of Chaetognaths". Current Biology 16 (15): R593–R596. doi:10.1016/j.cub.2006.07.006. PMID 16890517. Bibcode: 2006CBio...16.R593B.
- ↑ Terazaki, M.; Miller, C. B. (1982-11-01). "Reproduction of meso- and bathypelagic chaetognaths in the genus Eukrohnia" (in en). Marine Biology 71 (2): 193–196. doi:10.1007/BF00394629. ISSN 1432-1793. Bibcode: 1982MarBi..71..193T.
- ↑ 14.0 14.1 Daponte, M.C; Capitanio, F.L; Nahabedian, D.E; Viñas, M.D; Negri, R.M (2004). "Sagitta friderici Ritter-Záhony (Chaetognatha) from South Atlantic waters: Abundance, population structure, and life cycle". ICES Journal of Marine Science 61 (4): 680–686. doi:10.1016/j.icesjms.2004.03.006. Bibcode: 2004ICJMS..61..680D.
- ↑ Kosobokova, Ksenia N.; Hopcroft, Russell R. (2010). "Diversity and vertical distribution of mesozooplankton in the Arctic's Canada Basin". Deep Sea Research Part II: Topical Studies in Oceanography 57 (1–2): 96–110. doi:10.1016/j.dsr2.2009.08.009. Bibcode: 2010DSRII..57...96K.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 Choo, Seohwi; Jeong, Man-Ki; Soh, Ho Young (2022). "Taxonomic reassessment of chaetognaths (Chaetognatha, Sagittoidea, Aphragmophora) from Korean waters". ZooKeys (1106): 165–211. doi:10.3897/zookeys.1106.80184. PMID 36760822. Bibcode: 2022ZooK.1106..165C.
- ↑ Sullivan, Barbara K. (1980). "In situ feeding behavior of Sagitta elegans and Eukrohnia hamata (Chaetognatha) in relation to the vertical distribution and abundance of prey at Ocean Station "P"1". Limnology and Oceanography 25 (2): 317–326. doi:10.4319/lo.1980.25.2.0317. Bibcode: 1980LimOc..25..317S.
- ↑ Grigor, Jordan J.; Schmid, Moritz S.; Caouette, Marianne; St.-Onge, Vicky; Brown, Thomas A.; Barthélémy, Roxane-M. (2020-07-01). "Non-carnivorous feeding in Arctic chaetognaths" (in en). Progress in Oceanography 186. doi:10.1016/j.pocean.2020.102388. ISSN 0079-6611. Bibcode: 2020PrOce.18602388G. http://www.sciencedirect.com/science/article/pii/S0079661120301270.
- ↑ Thuesen, Erik V. (1991). "The Tetrodotoxin Venom of Chaetognaths". The Biology of chaetognaths. Oxford University Press. pp. 55–60. ISBN 978-0-19-857715-7.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 Piovani, Laura; Gavriouchkina, Daria; Parey, Elise; Sarre, Luke A.; Peijnenburg, Katja T. C. A.; Martín-Durán, José María; Rokhsar, Daniel S.; Satoh, Noriyuki et al. (2025). "The genomic origin of the unique chaetognath body plan". Nature. doi:10.1038/s41586-025-09403-2. PMID 40804517.
- ↑ 21.0 21.1 21.2 21.3 Papillon, Daniel; Perez, Yvan; Caubit, Xavier; Le Parco, Yannick (2004). "Identification of Chaetognaths as Protostomes is Supported by the Analysis of Their Mitochondrial Genome". Molecular Biology and Evolution 21 (11): 2122–2129. doi:10.1093/molbev/msh229. PMID 15306659.
- ↑ Formaggioni, Alessandro; Luchetti, Andrea; Plazzi, Federico (2021). "Mitochondrial Genomic Landscape: A Portrait of the Mitochondrial Genome 40 Years after the First Complete Sequence". Life 11 (7): 663. doi:10.3390/life11070663. PMID 34357035. Bibcode: 2021Life...11..663F.
- ↑ Helfenbein, Kevin G.; Fourcade, H. Matthew; Vanjani, Rohit G.; Boore, Jeffrey L. (2004). "The mitochondrial genome of Paraspadella gotoi is highly reduced and reveals that chaetognaths are a sister group to protostomes". Proceedings of the National Academy of Sciences 101 (29): 10639–10643. doi:10.1073/pnas.0400941101. PMID 15249679. Bibcode: 2004PNAS..10110639H.
- ↑ Marlétaz, Ferdinand; Le Parco, Yannick; Liu, Shenglin; Peijnenburg, Katja TCA (2017). "Extreme Mitogenomic Variation in Natural Populations of Chaetognaths". Genome Biology and Evolution 9 (6): 1374–1384. doi:10.1093/gbe/evx090. PMID 28854623.
- ↑ Bekkouche, Nicolas; Gąsiorowski, Ludwik (2022). "Careful amendment of morphological data sets improves phylogenetic frameworks: re-evaluating placement of the fossil Amiskwia sagittiformis". Journal of Systematic Palaeontology 20: 1–14. doi:10.1080/14772019.2022.2109217. https://hal.sorbonne-universite.fr/hal-03828531/file/Manuscript_Amiskwia_Bekkouche_and_Gasiorowski_Hall_version_compressed.pdf.
- ↑ Systema Naturae 2000 Taxon: Phylum Chaetognatha per Margulis and Schwartz (select Margulis & Schwartz in 'Classification by')—last retrieved November 25, 2006
- ↑ Marlétaz, Ferdinand; Peijnenburg, Katja T. C. A.; Goto, Taichiro; Satoh, Noriyuki; Rokhsar, Daniel S. (2019). "A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans". Current Biology 29 (2): 312–318.e3. doi:10.1016/j.cub.2018.11.042. PMID 30639106. Bibcode: 2019CBio...29E.312M. https://discovery.ucl.ac.uk/10097250/1/Phylogeny_chaeto_rv9s_pre.pdf.
- ↑ Vinther, Jakob; Parry, Luke A. (2019). "Bilateral jaw elements in Amiskwia sagittiformis bridge the morphological gap between gnathiferans and chaetognaths". Current Biology 29 (5): 881–888.e1. doi:10.1016/j.cub.2019.01.052. PMID 30799238. Bibcode: 2019CBio...29E.881V. https://research-information.bris.ac.uk/files/183275665/Collated_for_pure.pdf.
- ↑ Fröbius, Andreas C.; Funch, Peter (2017). "Rotiferan Hox genes give new insights into the evolution of metazoan bodyplans". Nature Communications 8 (1): 9. doi:10.1038/s41467-017-00020-w. PMID 28377584. Bibcode: 2017NatCo...8....9F.
- ↑ Laumer, Christopher E.; Bekkouche, Nicolas; Kerbl, Alexandra; Goetz, Freya; Neves, Ricardo C.; Sørensen, Martin V.; Kristensen, Reinhardt M.; Hejnol, Andreas et al. (2015). "Spiralian phylogeny informs the evolution of microscopic lineages". Current Biology 25 (15): 2000–2006. doi:10.1016/j.cub.2015.06.068. PMID 26212884. Bibcode: 2015CBio...25.2000L.
- ↑ Sielaff, Malte; Schmidt, Hanno; Struck, Torsten H.; Rosenkranz, David; Mark Welch, David B.; Hankeln, Thomas; Herlyn, Holger (2016). "Phylogeny of Syndermata (syn. Rotifera): Mitochondrial gene order verifies epizoic Seisonidea as sister to endoparasitic Acanthocephala within monophyletic Hemirotifera". Molecular Phylogenetics and Evolution 96: 79–92. doi:10.1016/j.ympev.2015.11.017. PMID 26702959. Bibcode: 2016MolPE..96...79S.
- ↑ Systema Naturae 2000 Taxon: Phylum Chaetognatha per Cavalier-Smith (select Cavalier-Smith in 'Classification by')—last retrieved November 25, 2006
- ↑ "Broad taxon and gene sampling indicate that chaetognaths are protostomes". Current Biology 16 (15): R575–R576. August 2006. doi:10.1016/j.cub.2006.07.017. PMID 16890509. Bibcode: 2006CBio...16.R575M.
- ↑ "Chaetognath phylogenomics: a protostome with deuterostome-like development". Current Biology 16 (15): R577-8. August 2006. doi:10.1016/j.cub.2006.07.016. PMID 16890510. Bibcode: 2006CBio...16.R577M.
- ↑ "Identification of chaetognaths as protostomes is supported by the analysis of their mitochondrial genome". Molecular Biology and Evolution 21 (11): 2122–9. November 2004. doi:10.1093/molbev/msh229. PMID 15306659.
- ↑ "Immunohistochemical analysis and 3D reconstruction of the cephalic nervous system in Chaetognatha: Insights into the evolution of an early bilaterian brain?". Invertebrate Biology 129 (1): 77–104. February 2010. doi:10.1111/j.1744-7410.2010.00189.x.
- ↑ "A new look at the ventral nerve centre of Sagitta: implications for the phylogenetic position of Chaetognatha (arrow worms) and the evolution of the bilaterian nervous system". Frontiers in Zoology 4: 14. May 2007. doi:10.1186/1742-9994-4-14. PMID 17511857.
- ↑ "Rotiferan Hox genes give new insights into the evolution of metazoan bodyplans". Nature Communications 8 (1): 9. April 2017. doi:10.1038/s41467-017-00020-w. PMID 28377584. Bibcode: 2017NatCo...8....9F.
- ↑ "A New Spiralian Phylogeny Places the Enigmatic Arrow Worms among Gnathiferans" (in en). Current Biology 29 (2): 312–318.e3. January 2019. doi:10.1016/j.cub.2018.11.042. PMID 30639106. Bibcode: 2019CBio...29E.312M. https://discovery.ucl.ac.uk/10097250/1/Phylogeny_chaeto_rv9s_pre.pdf.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedVannier2007 - ↑ "A possible Lower Cambrian chaetognath (arrow worm)". Science 298 (5591): 187. October 2002. doi:10.1126/science.1075059. PMID 12364798.
- ↑ "Taphonomy and palaeoecology of the Early Cambrian Chengjiang Biota from Eastern Yunnan, China". Berliner Paläobiologische Abhandlungen 7: 1–197. 2005.
- ↑ Park, Tae-Yoon S.; Nielsen, Morten Lunde; Parry, Luke A.; Sørensen, Martin Vinther; Lee, Mirinae; Kihm, Ji-Hoon; Ahn, Inhye; Park, Changkun et al. (2024). "A giant stem-group chaetognath". Science Advances 10 (1). doi:10.1126/sciadv.adi6678. PMID 38170772. Bibcode: 2024SciA...10I6678P.
- ↑ Vinther, Jakob; Parry, Luke A.; Lee, Mirinae; Nielsen, Morten Lunde; Oh, Yeongju; Park, Changkun; Kihm, Ji-Hoon; DeVivo, Giacinto et al. (2025-07-25). "A fossilized ventral ganglion reveals a chaetognath affinity for Cambrian nectocaridids" (in en). Science Advances 11 (30). doi:10.1126/sciadv.adu6990. ISSN 2375-2548. PMC 12285702. https://www.science.org/doi/10.1126/sciadv.adu6990.
- ↑ "New evidence for the protoconodont origin of chaetognaths". Acta Palaeontologica Polonica 47 (3): 405–419. 2002. http://www.app.pan.pl/archive/published/app47/app47-405.pdf.
- ↑ Vannier, J.; Steiner, M.; Renvoisé, E.; Hu, S.-X; Casanova, J.-P (2007). "Early Cambrian origin of modern food webs: Evidence from predator arrow worms". Proceedings of the Royal Society B: Biological Sciences 274 (1610): 627–633. doi:10.1098/rspb.2006.3761. PMID 17254986.
- ↑ Slabber, Martinus (1778). Natuurkundige Verlustigingen, Behelzende Microscopise Waarneemingen Van de in—En Uitlandse Water—En Land-Dieren. Haarlem: J. Bosch. pp. 46–48. https://books.google.com/books?id=f8rDmjs4abkC&pg=PA3.
- ↑ Quoy, J.R.C.; Gaimard, J.P. "Observations Zoologiques Faites à Bord de l'Astrolabe, en Mai 1826, dans le Détroit de Gibraltar (suite et fin). Description des genres Biphore, Carinaire, Hyale, Flèche, Cléodore, Anatife et Briarée." Ann. Sci. Nat. 1827, 10, 225–239
- ↑ Darwin, C. "Observations on the Structure and Propagation of the Genus Sagitta." Ann. Mag. Nat. Hist. 1844, 13, 1–6.
- ↑ Krohn, M.A. (1845). "XXXI.— Anatomical and physiological observations on Sagitta bipunctata". Annals and Magazine of Natural History 16 (106): 289–304. doi:10.1080/037454809496523. https://www.biodiversitylibrary.org/part/60372.
- ↑ Leuckart, R. Nachträge und Berichtigungen zu dem ersten Bande von J. van Der Hoeven's Handbuch der Zoologie. Eine Systematisch Geordnete Übersicht der Hauptsächlichste Neueren Leistungen:über die Zoologie der Wirbellosen Thiere; L. Voss: Leipzig, Germany, 1856. (In German)
- ↑ Ritter-Záhony R. (1911) "Revision der Chaetognathan." Deutsche Sudpolar Expedition 1901–1903. Band 13, Zoologie 5. Hft. 1: 1–72.
- ↑ Tokioka, Takasi (1965). "The Taxonomical Outline of Chaetognatha". Publications of the Seto Marine Biological Laboratory 12 (5): 335–357. doi:10.5134/175381.
- ↑ Bieri, Robert. "Systematics of the Chaetognatha." in The biology of chaetognaths (1991): 122–136.
- ↑ San Martin, Carmen, ed (2018-09-19). "Ultrastructure of Meelsvirus: A nuclear virus of arrow worms (phylum Chaetognatha) producing giant "tailed" virions". PLOS ONE 13 (9). doi:10.1371/journal.pone.0203282. PMID 30231047. Bibcode: 2018PLoSO..1303282S.
- ↑ "Prey detection by Chaetognatha via a vibration sense". Proceedings of the Royal Society of London. Series B. Biological Sciences 168 (1013): 413–419. 1967-11-14. doi:10.1098/rspb.1967.0072. Bibcode: 1967RSPSB.168..413H.
- ↑ "Ultrastructural study and ontogenesis of the appendages and related musculature of Paraspadella (Chaetognatha)". Tissue & Cell 35 (5): 339–51. October 2003. doi:10.1016/S0040-8166(03)00055-7. PMID 14517101.
- ↑ Roxane-Marie Barthélémy, Eric Faure, Taichiro Goto: Serendipitous Discovery in a Marine Invertebrate (Phylum Chaetognatha) of the Longest Giant Viruses Reported till Date. In: Biology, 2019, Abstract
External links
- Image of Pseudosagitta gazellae with a krill in its gut from the Tasmanian Aquaculture and Fisheries Institute
- Chaetognatha of the World – last retrieved December 13, 2006
- Eric Fauré, Roxane-Marie Barthélémy: Specific mitochondrial ss-tRNAs in phylum Chaetognatha. In: Journal of Entomology and Zoology Studies 7(3), April 2019, pp. 304–315. hal-02130653
Wikidata ☰ {{{from}}} entry
 |

