Chemistry:Guanabenz
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Wytensin |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a686003 |
| Pregnancy category |
|
| ATC code |
|
| Pharmacokinetic data | |
| Protein binding | 90% |
| Elimination half-life | 6 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
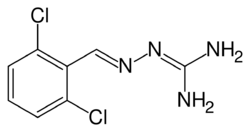
| Formula | C8H8Cl2N4 |
| Molar mass | 231.08 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Guanabenz (pronounced GWAHN-a-benz, sold under the trade name Wytensin) is an alpha agonist that is selective to the alpha-2 adrenergic receptor. Guanabenz is used as an antihypertensive drug used in the treatment of high blood pressure (hypertension).[1][2]
The most common side effects during guanabenz therapy are dizziness, drowsiness, dry mouth, headache and weakness.[3]
Guanabenz can make one drowsy or less alert, therefore driving or operating dangerous machinery is not recommended.
Research
Guanabenz also has some anti-inflammatory properties in different pathological situations, including multiple sclerosis.[4]
Guanabenz was found in one study to exert an inhibitory effect by decreasing the abundance of the enzyme CH25H, a cholesterol hydroxylase linked to antiviral immunity. Therefore, it is suggested that the drug and similar compounds could be used to treat type I interferon-dependent pathologies and that the CH25H enzyme could be a therapeutic target to control these diseases,[5] including amyotrophic lateral sclerosis.
See also
References
- ↑ "Comparative antihypertensive effects of guanabenz and clonidine". The Journal of International Medical Research 10 (1): 6–14. 1982. doi:10.1177/030006058201000102. PMID 7037502.[yes|permanent dead link|dead link}}]
- ↑ "Studies on the mechanism of the central antihypertensive effect of guanabenz and clonidine". Journal of Hypertension Supplement 2 (3): S543–S546. December 1984. PMID 6599714. http://ukpmc.ac.uk/abstract/MED/6599714.[yes|permanent dead link|dead link}}]
- ↑ "Guanabenz | The Merck Index Online". https://www.rsc.org/Merck-Index/monograph/m5863/guanabenz?q=unauthorize.
- ↑ "Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic". Nature Communications 6: 6532. March 2015. doi:10.1038/ncomms7532. PMID 25766071. Bibcode: 2015NatCo...6.6532W.
- ↑ "Guanabenz inhibits TLR9 signaling through a pathway that is independent of eIF2α dephosphorylation by the GADD34/PP1c complex". Science Signaling 11 (514): eaam8104. January 2018. doi:10.1126/scisignal.aam8104. PMID 29363586.
 |
