Chemistry:Doxazosin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /dɒkˈseɪzəsɪn/ dok-SAY-zə-sin OR /ˌdɒksəˈzoʊsɪn/ DOK-sə-ZOH-sin |
| Trade names | Cardura, Carduran, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693045 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 65% |
| Protein binding | 98% |
| Metabolism | Liver |
| Elimination half-life | 22 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
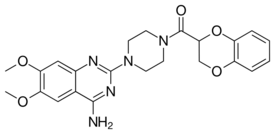
| Formula | C23H25N5O5 |
| Molar mass | 451.483 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Doxazosin, sold under the brand names Cardura among others, is a medication used to treat symptoms of benign prostatic hyperplasia (enlarged prostate) and hypertension (high blood pressure).[1][2] For high blood pressure, it is a less preferred option.[2] It is taken by mouth.[2]
Common side effects include dizziness, sleepiness, swelling, nausea, shortness of breath, and abdominal pain.[2] Severe side effects may include low blood pressure with standing, an irregular heart beat, and priapism.[2][3] It is a α1-selective adrenergic blocker in the quinazoline class of compounds.[2]
Doxazosin was patented in 1977 and came into medical use in 1988.[4] It is available as a generic medication.[3] In 2020, it was the 209th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[5][6]
A 2021 study associated doxazosin with decelerated biological aging in humans and confirmed its causal role in longevity in C. elegans.[7]
Medical uses
High blood pressure
Doxazosin is usually added to other antihypertensive therapy such as calcium channel antagonists, diuretics, beta-adrenoreceptor antagonists, angiotensin-converting enzyme inhibitors and angiotensin-2 receptor blockers.[8]
Doxazosin is generally considered to be safe, well tolerated and effective as an add-on antihypertensive drug.[9]
Like other alpha-1 receptor antagonists, it has a role in the peri-operative management of pheochromocytoma.[10]
Benign prostatic hyperplasia
Doxazosin is considered to be effective in reducing urinary symptom scores and improving peak urinary flow in men with benign prostatic hypertrophy.[11]
History
The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) study stopped its arm of the trial looking at alpha blockers, because doxazosin was less effective than a simple diuretic, and because patients on doxazosin had a 25% higher rate of cardiovascular disease and twice the rate of congestive heart failure as patients on diuretics.[12] Pfizer, aware of the results before publication, launched a marketing campaign in early 2000, and sales were largely unaffected, despite the dangers highlighted by the study.[13][14]
References
- ↑ 1.0 1.1 "Cardura- doxazosin mesylate tablet". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eefc47bc-c10f-49bb-8ef7-5999f798ab39.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Doxazosin Mesylate Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/doxazosin-mesylate.html.
- ↑ 3.0 3.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 765. ISBN 9780857113382.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 455. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA455.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Doxazosin - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Doxazosin.
- ↑ "Biological Age Prediction From Wearable Device Movement Data Identifies Nutritional and Pharmacological Interventions for Healthy Aging". Frontiers in Aging 2: 708680. 2021. doi:10.3389/fragi.2021.708680. PMID 35822021.
- ↑ "Doxazosin in the current treatment of hypertension". Expert Opinion on Pharmacotherapy 9 (4): 625–633. March 2008. doi:10.1517/14656566.9.4.625. PMID 18312163.
- ↑ "Time to re-appraise the role of alpha-1 adrenoceptor antagonists in the management of hypertension?". Journal of Hypertension 28 (9): 1796–1803. September 2010. doi:10.1097/HJH.0b013e32833b912c. PMID 20543713. https://lirias.kuleuven.be/handle/123456789/269628.
- ↑ "Anti-hypertensive treatment in pheochromocytoma and paraganglioma: current management and therapeutic features". Endocrine 45 (3): 469–478. April 2014. doi:10.1007/s12020-013-0007-y. PMID 23817839.
- ↑ "The efficacy and safety of alpha-1 blockers for benign prostatic hyperplasia: an overview of 15 systematic reviews". Current Medical Research and Opinion 29 (3): 279–287. March 2013. doi:10.1185/03007995.2013.766594. PMID 23323875.
- ↑ "Validation of Heart Failure Events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants Assigned to Doxazosin and Chlorthalidone". Current Controlled Trials in Cardiovascular Medicine 3 (1): 10. November 2002. doi:10.1186/1468-6708-3-10. PMID 12459039.
- ↑ Goldacre, Ben (2012) Bad Pharma How drug companies mislead doctors and harm patients, Fourth Estate, ISBN:0007350740.
- ↑ "Spin doctors soft pedal data on antihypertensives". BMJ 326 (7381): 170. 2003-01-18. doi:10.1136/bmj.326.7381.170.
External links
- "Doxazosin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/doxazosin.
{{Navbox
| name = Drugs used in benign prostatic hypertrophy | title = Drugs used in [[Medicine:Benign prostatic hyperpbenign prostatic hyperplasia (G04C) | state = collapsed | listclass = hlist
| group1 = 5α-Reductase inhibitors | list1 =
| group2 = Alpha-1 blockers | list2 =
| group3 = Steroidal antiandrogens | list3 =
| group4 = Herbal products | list4 =
| group5 = Others | list5 =
}}
 |

