Chemistry:Einsteinium(III) iodide
From HandWiki
 | |

| |
| Names | |
|---|---|
| IUPAC names
Einsteinium triiodide
Einsteinium(III) iodide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| EsI3 | |
| Molar mass | 632.796 g/mol |
| Appearance | amber-coloured solid, glows red in the dark[1] |
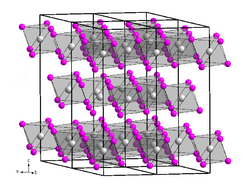
| Structure | |
| Hexagonal | |
| R3 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Einsteinium triiodide is an iodide of the synthetic actinide einsteinium which has the molecular formula EsI3. This crystalline salt is an amber-coloured solid.[1] It glows red in the dark due to einsteinium's intense radioactivity.
It crystallises in the hexagonal crystal system in the space group R3 with the lattice parameters a = 753 pm and c = 2084.5 pm with six formula units per unit cell. Its crystal structure is isotypic with that of bismuth(III) iodide.[2][3]
References
- ↑ 1.0 1.1 Arnold F. Holleman, Nils Wiberg: Lehrbuch der Anorganischen Chemie, 102nd Edition, de Gruyter, Berlin 2007, ISBN:978-3-11-017770-1, p. 1969.
- ↑ R. G. Haire, ORNL Report 5485, 1978.
- ↑ J. R. Peterson: "Chemical Properties of Einsteinium: Part II", in: G. T. Seaborg (ed.): Proceedings of the 'Symposium Commemorating the 25th Anniversary of Elements 99 and 100', 23. January 1978; Report LBL-7701, April 1979, pp. 55–64.
Further reading
- Haire, Richard G. (2006). "Einsteinium". in Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements. 3 (3rd ed.). Dordrecht, the Netherlands: Springer. pp. 1577–1620. doi:10.1007/1-4020-3598-5_12. http://radchem.nevada.edu/classes/rdch710/files/einsteinium.pdf.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |
