Chemistry:Caesium iodide
 CsI crystal
| |
 Scintillating CsI crystal
| |
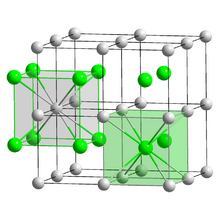 Crystal structure
| |
| |
| Names | |
|---|---|
| IUPAC name
Caesium iodide
| |
| Other names
Cesium iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| CsI | |
| Molar mass | 259.809 g/mol[2] |
| Appearance | white crystalline solid |
| Density | 4.51 g/cm3[2] |
| Melting point | 632 °C (1,170 °F; 905 K)[2] |
| Boiling point | 1,280 °C (2,340 °F; 1,550 K)[2] |
| 848 g/L (25 °C)[2] | |
| −82.6·10−6 cm3/mol[3] | |
Refractive index (nD)
|
1.9790 (0.3 µm) 1.7873 (0.59 µm) 1.7694 (0.75 µm) 1.7576 (1 µm) 1.7428 (5 µm) 1.7280 (20 µm)[4] |
| Structure | |
| CsCl, cP2 | |
| Pm3m, No. 221[5] | |
a = 0.4503 nm
| |
Lattice volume (V)
|
0.0913 nm3 |
Formula units (Z)
|
1 |
| Cubic (Cs+) Cubic (I−) | |
| Thermochemistry | |
Heat capacity (C)
|
52.8 J/mol·K[6] |
Std molar
entropy (S |
123.1 J/mol·K[6] |
Std enthalpy of
formation (ΔfH⦵298) |
−346.6 kJ/mol[6] |
Gibbs free energy (ΔfG˚)
|
−340.6 kJ/mol[6] |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H315, H317, H319, H335 | |
| P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+312, P302+352, P304+340, P305+351+338, P308+313, P312, P321, P330, P332+313, P333+313, P337+313, P362, P363, P391, P403+233 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2386 mg/kg (oral, rat)[1] |
| Related compounds | |
Other anions
|
Caesium fluoride Caesium chloride Caesium bromide Caesium astatide |
Other cations
|
Lithium iodide Sodium iodide Potassium iodide Rubidium iodide Francium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Caesium iodide or cesium iodide (chemical formula CsI) is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.[7]
Synthesis and structure
Bulk caesium iodide crystals have the cubic CsCl crystal structure, but the structure type of nanometer-thin CsI films depends on the substrate material – it is CsCl for mica and NaCl for LiF, NaBr and NaCl substrates.[9]
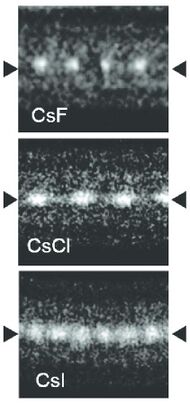
Caesium iodide atomic chains can be grown inside double-wall carbon nanotubes. In such chains I atoms appear brighter than Cs atoms in electron micrographs despite having a smaller mass. This difference was explained by the charge difference between Cs atoms (positive), inner nanotube walls (negative) and I atoms (negative). As a result, Cs atoms are attracted to the walls and vibrate more strongly than I atoms, which are pushed toward the nanotube axis.[8]
Properties
| Т (°C) | 0 | 10 | 20 | 25 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (wt%) | 30.9 | 37.2 | 43.2 | 45.9 | 48.6 | 53.3 | 57.3 | 60.7 | 63.6 | 65.9 | 67.7 | 69.2 |
Applications
An important application of caesium iodide crystals, which are scintillators, is electromagnetic calorimetry in experimental particle physics. Pure CsI is a fast and dense scintillating material with relatively low light yield that increases significantly with cooling,[11] and a fairly small Molière radius is 3.5 cm. It exhibits two main emission components: one in the near ultraviolet region at the wavelength of 310 nm and one at 460 nm. The drawbacks of CsI are a high temperature gradient and a slight hygroscopicity.
Caesium iodide is used as a beamsplitter in Fourier transform infrared (FTIR) spectrometers. It has a wider transmission range than the more common potassium bromide beamsplitters, working range into the far infrared. However, optical-quality CsI crystals are very soft and hard to cleave or polish. They should also be coated (typically with germanium) and stored in a desiccator, to minimize interaction with atmospheric water vapors.[12]
In addition to image intensifier input phosphors, caesium iodide is often also used in medicine as the scintillating material in flat panel x-ray detectors.[13]
References
- ↑ 1.0 1.1 Cesium iodide. U.S. National Library of Medicine
- ↑ 2.0 2.1 2.2 2.3 2.4 Haynes, p. 4.57
- ↑ Haynes, p. 4.132
- ↑ Haynes, p. 10.240
- ↑ Huang, Tzuen-Luh; Ruoff, Arthur L. (1984). "Equation of state and high-pressure phase transition of CsI". Physical Review B 29 (2): 1112. doi:10.1103/PhysRevB.29.1112. Bibcode: 1984PhRvB..29.1112H.
- ↑ 6.0 6.1 6.2 6.3 Haynes, p. 5.10
- ↑ Kowalski, M. P.; Fritz, G. G.; Cruddace, R. G.; Unzicker, A. E.; Swanson, N. (1986). "Quantum efficiency of cesium iodide photocathodes at soft x-ray and extreme ultraviolet wavelengths". Applied Optics 25 (14): 2440. doi:10.1364/AO.25.002440. PMID 18231513. Bibcode: 1986ApOpt..25.2440K.
- ↑ 8.0 8.1 Senga, Ryosuke; Komsa, Hannu-Pekka; Liu, Zheng; Hirose-Takai, Kaori; Krasheninnikov, Arkady V.; Suenaga, Kazu (2014). "Atomic structure and dynamic behaviour of truly one-dimensional ionic chains inside carbon nanotubes". Nature Materials 13 (11): 1050–4. doi:10.1038/nmat4069. PMID 25218060. Bibcode: 2014NatMa..13.1050S.
- ↑ Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica 4 (6): 487–489. doi:10.1107/S0365110X51001641. Bibcode: 1951AcCry...4..487S.
- ↑ Haynes, p. 5.191
- ↑ Mikhailik, V.; Kapustyanyk, V.; Tsybulskyi, V.; Rudyk, V.; Kraus, H. (2015). "Luminescence and scintillation properties of CsI: A potential cryogenic scintillator". Physica Status Solidi B 252 (4): 804–810. doi:10.1002/pssb.201451464. Bibcode: 2015PSSBR.252..804M.
- ↑ Sun, Da-Wen (2009). Infrared Spectroscopy for Food Quality Analysis and Control. Academic Press. pp. 158–. ISBN 978-0-08-092087-0. https://books.google.com/books?id=bOWUDeiYshsC&pg=PA158.
- ↑ Lança, Luís; Silva, Augusto (2012). "Digital Radiography Detectors: A Technical Overview". Digital Imaging Systems for Plain Radiography. Springer. doi:10.1007/978-1-4614-5067-2_2. ISBN 978-1-4614-5066-5. https://www.springer.com/cda/content/document/cda_downloaddocument/9781461450665-c1.pdf?SGWID=0-0-45-1368105-p174548440. Retrieved 2017-08-28.
Cited sources
- Haynes, William M., ed (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1439855110.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |
