Chemistry:Tislelizumab
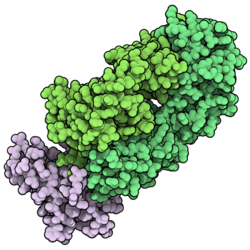 Fab fragment of tislelizumab (green) binding the extracellular domain of PD-1 (pale pink). From PDB entry 7BXA | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | PD-1 |
| Clinical data | |
| Trade names | Tevimbra |
| Other names | |
| Routes of administration | Intravenous |
| Drug class | Antineoplastic agent |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
Tislelizumab, sold under the brand name Tevimbra among others, is a humanized monoclonal antibody directed against PD-1.[5] It prevents PD-1 from binding to the ligands PD-L1 and PD-L2 (hence it is a checkpoint inhibitor). It is designed to bind less to Fc gamma receptors.[6] It is being developed by BeiGene (after a period with Celgene Corp).[7]
Medical uses
China
Tislelizumab was approved by China's National Medical Products Administration :
- in December 2019, to treat people with classical Hodgkin’s lymphoma (cHL) who have received at least two prior therapies[8]
- and in April 2020, to treat people with locally advanced or metastatic urothelial carcinoma (UC) with PD-L1 high expression whose disease progressed during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.[9]
History
Phase I trials began in the US and Australia in June 2015.[10] Some early results were announced in July 2016.[11][5]
A phase II clinical trial for urothelial cancer started in China in 2017.[6]
It is in a phase III trial for NSCLC.[12]
A multicenter phase III trial for advanced hepatocellular carcinoma started in January 2018.[7]
Pharmacokinetics
Phase I clinical trial results give an elimination half-life of 11 to 17 days.[5]
References
- ↑ "BeiGene's Therapy Candidate Appears to Eliminate Tumors in Half of Hodgkin's Patients in Phase 2 Trial". Lymphoma News Today (Philadelphia, Pennsylvania, United States: BioNews Services). 27 July 2018. https://lymphomanewstoday.com/2018/07/27/beigene-treatment-candidate-eliminates-some-hodgkins-lymphoma-tumors/.
- ↑ "3 Reasons BeiGene Needs to Be on Your Radar". July 3, 2018. https://www.fool.com/investing/2018/07/03/3-reasons-beigene-needs-to-be-on-your-investing-ra.aspx.
- ↑ "Tevimbra Product information". 19 September 2023. https://ec.europa.eu/health/documents/community-register/html/h1758.htm.
- ↑ "Tevimbra EPAR". 4 October 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/tevimbra.
- ↑ 5.0 5.1 5.2 "Meeting Library - Meeting Library". https://meetinglibrary.asco.org/record/125804/abstract.
- ↑ 6.0 6.1 "BeiGene (BGNE) Commences Pivotal Trial of PD-1 Antibody BGB-A317 in China in Patients with Urothelial Cancer". https://www.streetinsider.com/Corporate+News/BeiGene+(BGNE)+Commences+Pivotal+Trial+of+PD-1+Antibody+BGB-A317+in+China+in+Patients+with+Urothelial+Cancer/13068864.html.
- ↑ 7.0 7.1 LTD, BeiGene (2 January 2018). "BeiGene Initiates Global Phase 3 Trial of Anti-PD-1 Antibody Tislelizumab in Patients with Hepatocellular Carcinoma". http://www.globenewswire.com/news-release/2018/01/02/1276905/0/en/BeiGene-Initiates-Global-Phase-3-Trial-of-Anti-PD-1-Antibody-Tislelizumab-in-Patients-with-Hepatocellular-Carcinoma.html.
- ↑ "BeiGene scores first China OK with PD-1 — to be manufactured by Boehringer Ingelheim". 2020-01-02. https://endpts.com/beigene-scores-first-china-ok-with-pd-1-to-be-manufactured-by-boehringer-ingelheim/.
- ↑ "Ploughing through a crowded PD-(L)1 market, BeiGene loads up on promising lung cancer data". 2020-04-14. https://endpts.com/ploughing-through-a-crowded-pd-l1-market-beigene-loads-up-on-promising-lung-cancer-data/.
- ↑ Clinical trial number NCT02407990 for "Study of the Safety, Pharmacokinetics and Antitumor Activities of BGB-A317 in Subjects With Advanced Tumors" at ClinicalTrials.gov
- ↑ "Immunotherapy Trial's Early Results Show Activity in Solid Tumors". 26 July 2016. https://immuno-oncologynews.com/2016/07/26/beigene-presents-initial-clinical-data-showing-anti-cancer-activity-in-range-of-solid-tumors/.
- ↑ Clinical trial number NCT03358875 for "Comparison of Efficacy and Safety of Anti-PD-1 Antibody BGB-A317 Versus Docetaxel as Treatment in the Second- or Third-line Setting in Patients With NSCLC" at ClinicalTrials.gov
 |

