Chemistry:Uranium nitride
| |
| Names | |
|---|---|
| IUPAC name
Uranium nitride
| |
| Identifiers | |
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| U2N3 | |
| Molar mass | 518.078 g/mol |
| Appearance | crystalline solid |
| Density | 11300 kg·m−3, solid |
| Melting point | 900 to 1,100 °C (1,650 to 2,010 °F; 1,170 to 1,370 K) (decomposes to UN) |
| Boiling point | Decomposes |
| 0.08 g/100 ml (20 °C) | |
| Structure | |
| Hexagonal, hP5 | |
| P-3m1, No. 164 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uranium nitride is any of a family of several ceramic materials: uranium mononitride (UN), uranium sesquinitride (U2N3) and uranium dinitride (UN2). The word nitride refers to the −3 oxidation state of the nitrogen bound to the uranium.
Uranium nitride has been considered as a potential fuel for nuclear reactors. It is said to be safer, stronger, denser, more thermally conductive and having a higher temperature tolerance. Challenges to implementation of the fuel include a complex conversion route from enriched UF6, the need to prevent oxidation during manufacturing and the need to define and license a final disposal route. The necessity to use expensive, highly isotopically enriched 15N is a significant factor to overcome. This is necessary due to the (relatively) high neutron capture cross-section of the far-more-common 14N, which affects the neutron economy of a reactor.[2]
Synthesis
Carbothermic reduction
The common technique for generating UN is carbothermic reduction of uranium oxide (UO2) in a 2 step method illustrated below.[3][4]
- 3UO2 + 6C → 2UC + UO2 + 4CO (in argon, > 1450 °C for 10 to 20 hours)
- 4UC + 2UO2 +3N2 → 6UN + 4CO
Sol-gel
Sol-gel methods and arc melting of pure uranium under nitrogen atmosphere can also be used.[5]
Ammonolysis
Another common technique for generating UN2 is the ammonolysis of uranium tetrafluoride. Uranium tetrafluoride is exposed to ammonia gas under high pressure and temperature, which replaces the fluorine with nitrogen and generates hydrogen fluoride.[6] Hydrogen fluoride is a colourless gas at this temperature and mixes with the ammonia gas.
Hydriding-nitriding
An additional method of UN synthesis employs fabrication directly from metallic uranium. By exposing metallic uranium to hydrogen gas at temperatures in excess of 280 °C, UH3 can be formed.[7] Furthermore, since UH3 has a higher specific volume than the metallic phase, hybridation can be used to physically decompose otherwise solid uranium. Following hybridation, UH3 can be exposed to a nitrogen atmosphere at temperatures around 500 °C, thereby forming U2N3. By additional heating to temperatures above 1150 °C, the sesquinitride can then be decomposed to UN.
- 2U + 3H2 → 2UH3
- 2UH3 + 1.5N2 → U2N3
- U2N3 → UN + 0.5N2
Use of the isotope 15N (which constitutes around 0.37% of natural nitrogen) is preferable because the predominant isotope, 14N, has a significant neutron absorption cross section which affects neutron economy and, in particular, it undergoes an (n,p) reaction which produces significant amounts of radioactive 14C which would need to be carefully contained and sequestered during reprocessing or permanent storage.[8]
Decomposition
Each uranium dinitride complex is considered to have three distinct compounds present simultaneously because of decomposing of uranium dinitride (UN2) into uranium sesquinitride (U2N3), and then uranium mononitride (UN). Uranium dinitrides decompose to uranium mononitride by the following sequence of reactions:[9]
- 4UN2 → 2U2N3+ N2
- 2U2N3 → 4UN +N2
Decomposition of UN2 is the most common method for isolating uranium sesquinitride (U2N3).
Uses
Uranium mononitride is being considered as a potential fuel for generation IV reactors such as the Hyperion Power Module reactor created by Hyperion Power Generation.[10] It has also been proposed as nuclear fuel in some fast neutron nuclear test reactors. UN is considered superior because of its higher fissionable density, thermal conductivity, and melting temperature than the most common nuclear fuel, uranium oxide (UO2), while also demonstrating lower release of fission product gases and swelling, and decreased chemical reactivity with cladding materials.[11] It also has a superior mechanical, thermal, and radiation stability compared to standard metallic uranium fuel.[9][12] The thermal conductivity is on the order of 4-8 times higher than that of uranium dioxide, the most commonly used nuclear fuel, at typical operating temperatures. Increased thermal conductivity results in a smaller thermal gradient between inner and outer sections of the fuel,[8] potentially allowing for higher operating temperatures and reducing macroscopic restructuring of the fuel, which limits fuel lifetime.[4]
Molecular and crystal structure
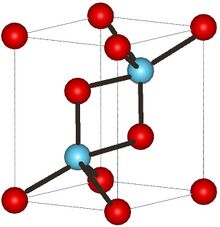
The uranium dinitride (UN2) compound has a face-centered cubic crystal structure of the calcium fluoride (CaF2) type with a space group of Fm3m.[13] Nitrogen forms triple bonds on each side of uranium forming a linear structure.[14][15]
α-(U2N3) has a body-centered cubic crystal structure of the (Mn2O3) type with a space group of Ia3 .[13]
UN has a face-centered cubic crystal structure of the NaCl type.[14][16] The metal component of the bond uses the 5f orbital of the uranium but forms a relatively weak interaction but is important for the crystal structure. The covalent portion of the bonds forms from the overlap between the 6d orbital and 7s orbital on the uranium and the 2p orbitals on the nitrogen.[14][17] N forms a triple bond with uranium creating a linear structure.[15]
Uranium nitrido derivatives
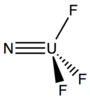
Recently, there have been many developments in the synthesis of complexes with terminal uranium nitride (–U≡N) bonds. In addition to radioactive concerns common to all uranium chemistry, production of uranium nitrido complexes has been slowed by harsh reaction conditions and solubility challenges. Nonetheless, syntheses of such complexes have been reported in the past few years, for example the three shown below among others.[18][19] Other U≡N compounds have also been synthesized or observed with various structural features, such as bridging nitride ligands in di-/polynuclear species, and various oxidation states.[20][21]
See also
- List of energy densities
- Nuclear fuel cycle
- Nuclear reactor
- Uranium carbide
- Uranium oxide
References
- ↑ R. B. Matthews; K. M. Chidester; C. W. Hoth; R. E. Mason; R. L. Petty (1988). "Fabrication and testing of uranium nitride fuel for space power reactors". Journal of Nuclear Materials 151 (3): 345. doi:10.1016/0022-3115(88)90029-3. Bibcode: 1988JNuM..151..345M.
- ↑ Chaudri, Khurrum Saleem (2013). "Coupled analysis for new fuel design using UN and UC for SCWR". Progress in Nuclear Energy 63: 57–65. doi:10.1016/j.pnucene.2012.11.001.
- ↑ Minato, Kazuo; Akabori, Mitsuo; Takano, Masahide; Arai, Yasuo; Nakajima, Kunihisa; Itoh, Akinori; Ogawa, Toru (2003). "Fabrication of nitride fuels for transmutation of minor actinides". Journal of Nuclear Materials 320 (1–2): 18–24. doi:10.1016/S0022-3115(03)00163-6. ISSN 0022-3115. Bibcode: 2003JNuM..320...18M.
- ↑ Jump up to: 4.0 4.1 Carmack, W. J. (2004). "Internal Gelation as Applied to the Production of Uranium Nitride Space Nuclear Fuel". AIP Conference Proceedings 699: 420–425. doi:10.1063/1.1649601. ISSN 0094-243X. Bibcode: 2004AIPC..699..420C.
- ↑ Ganguly, C.; Hegde, P. J. Sol-Gel Sci. Technol.. 1997, 9, 285.
- ↑ Silva, G. W. C.; Yeamans, C. B.; Ma, L.; Cerefice, G. S.; Czerwinski, K. R.; Sarrelberger, A. P. Chem. Mater.. 2008, 20, 3076.
- ↑ urn:nbn:se:kth:diva-35249: Manufacturing methods for (U-Zr)N-fuels
- ↑ Jump up to: 8.0 8.1 Matthews, R. B.; Chidester, K. M.; Hoth, C. W.; Mason, R. E.; Petty, R. L. Journal of Nuclear Materials. '1988, 151(3), 345.
- ↑ Jump up to: 9.0 9.1 Silva, G. W. Chinthaka; Yeamans, Charles B.; Sattelberger, Alfred P.; Hartmann, Thomas; Cerefice, Gary S.; Czerwinski, Kenneth R. (2009). "Reaction Sequence and Kinetics of Uranium Nitride Decomposition". Inorganic Chemistry 48 (22): 10635–10642. doi:10.1021/ic901165j. ISSN 0020-1669. PMID 19845318.
- ↑ Staff (2009-11-20). "Hyperion launches U2N3-fuelled, Pb-Bi-cooled fast reactor". Nuclear Engineering International (Global Trade Media, a division of Progressive Media Group Ltd.). https://www.neimagazine.com/news/newshyperion-launches-u2n3-fuelled-pb-bi-cooled-fast-reactor.
- ↑ "Simple method for producing a stable form of uranium nitride". Advanced Ceramics Report (International Newsletters). August 1, 2012. "[R]esearcher ... Stephen Liddle, says: '... it could help... extract and separate the 2-3% of the highly radioactive material in nuclear waste.'".
- ↑ Mizutani, A.; Sekimoto, H. Ann. Nucl. Energy. 2005, 25(9), 623–638.
- ↑ Jump up to: 13.0 13.1 Rundle, R. E.; Baenziger, N. C.; Wilson, A. S.; McDonald, R. A. J. Am. Chem Soc.. 1948, 70, 99.
- ↑ Jump up to: 14.0 14.1 14.2 Weck P. F., Kim E., Balakrishnan N., Poineau F., Yeamans C. B., and Czerwinski K. R. Chem. Phys. Lett.. 2007, 443, 82. doi:10.1016/j.cplett.2007.06.047
- ↑ Jump up to: 15.0 15.1 Wang, X.; Andrews, L.; Vlaisavljevich, B.; Gagliardi, L. Inorganic Chemistry. 2011, 50(8), 3826–3831. doi:10.1021/ic2003244
- ↑ Mueller, M. H.; Knott, H. W.Acta Crystallogr.. 1958, 11, 751–752. doi:10.1107/S0365110X58002061 ]
- ↑ Évarestov, R. A., Panin, A. I., & Losev, M. V. Journal of Structural Chemistry. 2008, 48, 125–135.
- ↑ Nocton, G.; Pécaut, J.; Mazzanti, M. A Nitrido-Centered Uranium Azido Cluster Obtained from a Uranium Azide. Angew. Chem. Int. Ed. 2008, 47 (16), 3040–3042. doi:10.1002/anie.200705742
- ↑ Thomson, R. K.; Cantat, T.; Scott, B. L.; Morris, D. E.; Batista, E. R.; Kiplinger, J. L. Uranium azide photolysis results in C–H bond activation and provides evidence for a terminal uranium nitride. Nature Chemistry 2010, 2, 723–729. doi:10.1038/nchem.705
- ↑ Fox, A. R.; Arnold, P. L.; Cummins, C. C. Uranium−Nitrogen Multiple Bonding: Isostructural Anionic, Neutral, and Cationic Uranium Nitride Complexes Featuring a Linear U═N═U Core. J. Am. Chem. Soc. 2010, 132 (10), 3250–3251. doi:10.1021/ja910364u
- ↑ Evans, W. J.; Kozimor, S. A.; Ziller, J. W. Molecular Octa-Uranium Rings with Alternating Nitride and Azide Bridges. Science 2005, 309 (5742), 1835–1838. doi:10.1126/science.1116452
- ↑ Fox, A.; Cummins, C. Uranium−Nitrogen Multiple Bonding: The Case of a Four-Coordinate Uranium(VI) Nitridoborate Complex. J. Am. Chem. Soc., 2009, 131 (16), 5716–5717. doi:10.1021/ja8095812
- ↑ Andrew, L.; Wang, X.; Lindh, R.; Roos, B.; Marsden, C. Simple N≡UF3 and P≡UF3 Molecules with Triple Bonds to Uranium. Angew. Chem. Int. Ed. 2008, 47 (29), 5366-5370. doi:10.1002/anie.200801120
- ↑ King, D.; Tuna, F.; McInnes, E.; McMaster, J.; Lewis, W.; Blake, A.; Liddle, S. T. Synthesis and Structure of a Terminal Uranium Nitride Complex. Science 2012, 337 (6095), 717–720. doi:10.1126/science.1223488
External links
- New Uranium Bond at The Periodic Table of Videos (University of Nottingham)