Chemistry:Chlorproethazine
| |
| Names | |
|---|---|
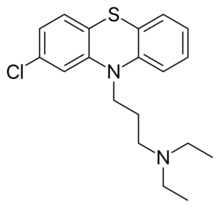
| Preferred IUPAC name
3-(2-Chlorophenothiazin-10-yl)-N,N-diethylpropan-1-amine | |
| Other names
RP-4909
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H23ClN2S | |
| Molar mass | 346.91732 g/mol |
| Pharmacology | |
| 1=ATC code }} | N05AA07 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorproethazine, sold under the brand name Neuriplege, is a drug of the phenothiazine group described as a muscle relaxant or tranquilizer which is or has been marketed in Europe as a topical cream for the treatment of muscle pain.[1][2][3][4][5] It has been associated with photoallergic contact dermatitis.[6][7]
Synthesis
Chlorproethazine can be synthesized from a diphenylsulfide derivative. The general scheme is sufficiently flexible to permit the interchange of the order of some of the steps.

Thus alkylation of 2-(2-bromo-phenylsulfanyl)-5-chloro-aniline [105790-02-1] (1) with 3-chloro-1-diethylaminopropane [104-77-8] (2) leads to the intermediate (3). Ring closure as above by nucleophilic aromatic displacement leads to the antipsychotic drug chlorproethazine (4).
The last step uses copper powder and is a form of the Ullmann condensation (i.e. the Goldberg reaction).
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 264–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA264.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 222–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA222.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 74–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA74.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 989–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA989.
- ↑ "Chlorproethazine". Drugs.com. https://www.drugs.com/international/chlorproethazine.html.
- ↑ Fisher's Contact Dermatitis. PMPH-USA. 2008. pp. 249–. ISBN 978-1-55009-378-0. https://books.google.com/books?id=dQBAzfyCeQ8C&pg=PA249.
- ↑ Contact Dermatitis. Springer Science & Business Media. 29 September 2010. pp. 373–. ISBN 978-3-642-03827-3. https://books.google.com/books?id=sSHIlWSOiroC&pg=PA373.
- ↑ Buisson P, Gailliot P, "Preparation of Phenothiazine Compounds", US patent 2769002, issued 30 October 1956, assigned to Rhône-Poulenc
 |

