Chemistry:Hydromorphinol
 | |
| Clinical data | |
|---|---|
| Other names | Hydromorphinol, 14-hydroxy-7,8-dihydromorphine, RAM-320 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H21NO4 |
| Molar mass | 303.358 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
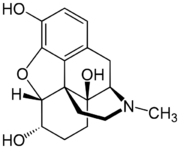
Hydromorphinol (RAM-320, 14-Hydroxydihydromorphine),[1] is an opiate analogue that is a derivative of morphine, where the 14-position has been hydroxylated and the 7,8- double bond saturated.[2] It has similar effects to morphine such as sedation, analgesia and respiratory depression, but is twice as potent as morphine[1] and has a steeper dose-response curve and longer half-life.[3] It is used in medicine as the bitartrate salt (free base conversion ratio 0.643, molecular weight 471.5) and hydrochloride (free base conversion ratio 0.770, molecular weight 393.9)
Hydromorphinol has also been discovered to occur naturally in trace amounts within opium, although this is a very rare occurrence.[4]
It is also called α-Oxymorphol, and oxymorphol is itself a mixture of hydromorphinol and 4,5α-Epoxy-17-methylmorphinan-3,6β,14-triol, β-Oxymorphol, which is different at position 6 on the morphine carbon skeleton.
Hydromorphinol was developed in Austria in 1932. In the United States , it was never available and is classified as a Schedule I drug with a DEA ACSCN of 9301. The salts in use are the bitartrate (free base conversion ratio 0.643) and hydrochloride (0.770). The 2014 national aggregate manufacturing quota was 2 grams, unchanged from prior years.[5]
Hydromorphinol is metabolised mainly in the liver in the same fashion as many other opioids and is itself a minor active metabolite of 14-Hydroxydihydrocodeine, an uncommonly used opiate (but is therefore also an active metabolite of a first-order active metabolite of oxycodone).
It is distributed under the trade name Numorphan in some countries. It is controlled under the Single Convention On Narcotic Drugs.
See also
- Oxymorphol
- N-Phenethylhydromorphinol (RAM-378)
References
- ↑ 1.0 1.1 Weiss U, "Morphine derivative", US patent 2960505, published 11/15/1960
- ↑ "Derivatives of Morphine. IV.114-Hydroxymorphine and 14-Hydroxydihydromorphine". Journal of Medicinal Chemistry 8: 123–5. January 1965. doi:10.1021/jm00325a028. PMID 14287245.
- ↑ "Influence of polarity on dose-response relationships of intrathecal opioids in rats". Pain 40 (3): 339–47. March 1990. doi:10.1016/0304-3959(90)91131-2. PMID 2326098.
- ↑ Ginsburg, David (1959). "Some recent advances in the chemistry of the opium alkaloids". UNODC Bulletin on Narcotics (3). http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1959-01-01_3_page002.html.
- ↑ "Quotas 2014". DEA Diversion Control Division. http://www.deadiversion.usdoj.gov/fed_regs/quotas/2014/fr0825.htm.
 |
