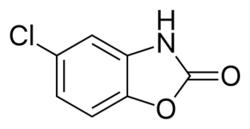
Chemistry:Chlorzoxazone
 | |
| Clinical data | |
|---|---|
| Trade names | Lorzone, Paraflex, Muscol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682577 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Skeletal muscle relaxants |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Well absorbed |
| Protein binding | 13–18% |
| Metabolism | Hepatic |
| Elimination half-life | 1.1 hours |
| Duration of action | 3–4 hours |
| Excretion | urine (<1%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C7H4ClNO2 |
| Molar mass | 169.56 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Chlorzoxazone (INN) is a centrally acting muscle relaxant used to treat muscle spasm and the resulting pain or discomfort. It can also be administered for acute pain in general and for tension headache (muscle contraction headache). It acts on the spinal cord by depressing reflexes. It is sold under the brand names Lorzone, Paraflex and Muscol and in combination form as Parafon Forte, a combination of chlorzoxazone and acetaminophen (paracetamol). Possible side effects include dizziness, lightheadedness, malaise, nausea, vomiting, and liver dysfunction. When used with acetaminophen it has added risk of hepatotoxicity.
It is available as a generic medication.[3]
Like metaxalone, its mechanism of action is still in question. It is believed that metaxalone works by altering serotonin levels and acting as a mild MAO inhibitor. The mechanism of action of chlorzoxazone is thought[by whom?] to act on GABAA and GABAB receptors and voltage-gated calcium channels to a degree. General central nervous system depression is the only currently accepted aspect to its medical benefits. Elucidation of the exact mechanism of action is ongoing but there is limited study due to the existence of more effective, safe muscle relaxants (e.g., diazepam, cyclobenzaprine, tizanidine), greatly limiting the potential benefit of identifying novel compounds which share chlorzoxazone's mechanism of action.
See also
References
- ↑ "Parafon DSC- chlorzoxazone tablet". 9 February 2010. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5af155d7-3780-435b-9a87-0ce01f1ed6ec.
- ↑ "Lorzone- chlorzoxazone tablet". 21 June 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bdd61b83-5bcd-4d23-8ef5-aeb9ca2f7c14.
- ↑ "Competitive Generic Therapy Approvals". 29 June 2023. https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals.
Further reading
- "Chlorzoxazone inhibit contraction of rat thoracic aorta". Eur J Pharmacol 545 (2–3): 161–6. 2006. doi:10.1016/j.ejphar.2006.06.063. PMID 16859676.
- "Effect of high-dose aspirin on CYP2E1 activity in healthy subjects measured using chlorzoxazone as a probe". J Clin Pharmacol 46 (1): 109–14. 2006. doi:10.1177/0091270005282635. PMID 16397290.
- "Chlorzoxazone metabolism is increased in fasted Sprague-Dawley rats". J Pharm Pharmacol 58 (1): 51–61. 2006. doi:10.1211/jpp.58.1.0007. PMID 16393464.
External links
- "Chlorzoxazone". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/chlorzoxazone.
- Chloroxazone Safety Data Sheet
- D.F. Marsh, U.S. Patent 2,895,877 (1959)
 |
