Chemistry:Caesium acetate
 Structural formula
| |||
 Unit cell of anhydrous caesium acetate.
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Caesium acetate | |||
| Other names
Cesium acetate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C2H3CsO2 | |||
| Molar mass | 191.949 g/mol | ||
| Appearance | colourless, hygroscopic | ||
| Density | 2.423 g/cm3, solid | ||
| Melting point | 194 °C (381 °F; 467 K) | ||
| Boiling point | 945 °C (1,733 °F; 1,218 K) | ||
| 945.1 g/100 g (−2.5 °C) 1345.5 g/100 ml (88.5 °C) | |||
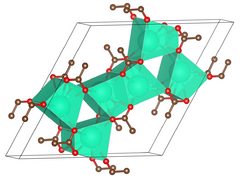
| Structure[2] | |||
| Primitve hexagonal | |||
| P6/m, No. 175 | |||
a = 1488.0 pm, c = 397.65 pm[2]
| |||
Lattice volume (V)
|
76.542 cm3·mol−1 | ||
Formula units (Z)
|
6 | ||
| Hazards | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Caesium formate | ||
Other cations
|
Lithium acetate Sodium acetate Potassium acetate Rubidium acetate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Caesium acetate or cesium acetate is an ionic caesium compound with the molecular formula CH3COOCs. It is a white solid that may be formed by the reaction of caesium hydroxide or caesium carbonate with acetic acid.[3]
Uses
It is used in organic synthesis. One example is in the Perkin synthesis: the formation of unsaturated cinnamic-type acids by the condensation of aromatic aldehydes with fatty acids. Replacement of the commonly used sodium acetate with caesium acetate has been shown to improve yields by up to 10 times.[3][4]
It is often used to invert secondary alcohols, first by direct SN2 substitution of the hydroxyl group with acetate, which is then converted back to a hydroxyl group.[3]
Caesium acetate is occasionally used instead of caesium formate in petroleum drilling fluids.[citation needed]
References
- ↑ Weast, Robert C., ed (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-91. ISBN 0-8493-0462-8..
- ↑ 2.0 2.1 Lossin, Adalbert; Meyer, Gerd (1993). "Kristallstruktur von Caesiumacetat, Cs(CH3COO)". Zeitschrift für Anorganische und Allgemeine Chemie 619 (8): 1462–1464. doi:10.1002/zaac.19936190823.
- ↑ 3.0 3.1 3.2 Yode, Ryan (2015) (in en), Cesium Acetate, John Wiley & Sons, pp. 1–11, doi:10.1002/047084289x.rn01845, ISBN 978-0-470-84289-8, https://onlinelibrary.wiley.com/doi/abs/10.1002/047084289X.rn01845, retrieved 2020-07-21
- ↑ Koepp, E.; Vögtle, F. (1987), "Perkin-Synthese mit Cäsiumacetat", Synthesis 1987 (2): 177–179, doi:10.1055/s-1987-27880.
Further reading
- Torisawa, Yasuhiro; Okabe, Hiromitsu; Ikegami, Shiro (1984), "Efficient Inversions of Secondary Alcohols using Cesium Acetate and 18-Crown-6", Chem. Lett. 13 (9): 1555–56, doi:10.1246/cl.1984.1555.
External links
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |