Chemistry:Ytterbium(III) bromide
From HandWiki
| |
| Names | |
|---|---|
| Other names
ytterbium tribromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| YbBr3 | |
| Molar mass | 412.77 g/mol |
| Appearance | white crystalline |
| Melting point | 677 °C (1,251 °F; 950 K)[1] |
| Boiling point | 1,800 °C (3,270 °F; 2,070 K)[1] |
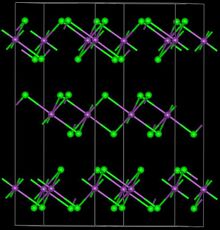
| Structure | |
| Trigonal, hR24 | |
| R-3, No. 148 | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ytterbium(III) bromide (YbBr3) is an inorganic chemical compound.
Refer to the adjacent table for the main properties of Ytterbium(III) bromide.
Preparation
Dissolving ytterbium oxide into 40% hydrobromic acid forms YbBr3·6H2O crystals. After mixing the hydrate with ammonium bromide and heating it in a vacuum, anhydrous YbBr3 can be obtained.[2]
- Yb2O3 + 6 HBr → 2 YbBr3 + 3 H2O
Ytterbium(III) bromide can also be prepared by directly heating ytterbium oxide and ammonium bromide.[3]
References
- ↑ 1.0 1.1 Walter Benenson; John W. Harris; Horst Stöcker (2002). Handbook of Physics. Springer. p. 781. ISBN 0-387-95269-1. https://books.google.com/books?id=RbLE77b6eRUC&pg=PA781.
- ↑ 林平娣, 吴国庆. 无水三溴化钐和三溴化镱的制备 [J]. 化学试剂, 1991(1):13-14.
- ↑ Gerd Meyer, Siegfried Dötsch, Thomas Staffel (1987). "The ammonium-bromide route to anhydrous rare earth bromides MBr3" (in en). Journal of the Less Common Metals 127: 155–160. doi:10.1016/0022-5088(87)90372-9. https://linkinghub.elsevier.com/retrieve/pii/0022508887903729. Retrieved 2020-05-29.
 |


