Chemistry:Ytterbium(II) chloride
From HandWiki
| |
| Names | |
|---|---|
| Other names
ytterbium dichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| YbCl2 | |
| Molar mass | 243.95 g/mol |
| Appearance | green crystals |
| Density | 5.27 g/cm3, solid |
| Melting point | 721 °C (1,330 °F; 994 K) |
| reacts[1] | |
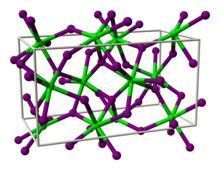
| Structure | |
| Orthorhombic, oP24 | |
| Pbca, No. 61 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ytterbium(II) chloride (YbCl2) is an inorganic chemical compound. It was first prepared in 1929 by W. K. Klemm and W. Schuth, by reduction of ytterbium(III) chloride, YbCl3, using hydrogen.
- 2 YbCl3 + H2 → 2 YbCl2 + 2 HCl
Like other Yb(II) compounds and other low-valence rare earth compounds, it is a strong reducing agent. It is unstable in aqueous solution, reducing water to hydrogen gas.[2]
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–94, ISBN 0-8493-0594-2
- ↑ ytterbium - Britannica Online Encyclopedia
 |

