Chemistry:Xanomeline
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
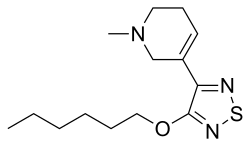
| Formula | C14H23N3OS |
| Molar mass | 281.42 g·mol−1 |
| 3D model (JSmol) | |
| |
Xanomeline (LY-246,708; Lumeron, Memcor) is a small molecule muscarinic acetylcholine receptor agonist that was first synthesized in a collaboration between Eli Lilly and Novo Nordisk as an investigational therapeutic being studied for the treatment of central nervous system disorders.[1][2]
Its pharmacological action is mediated primarily through stimulation of central nervous system muscarinic M1 and M4 receptor subtypes.[3][4] Xanomeline is currently being developed as a combination drug (Kar-XT; xanomeline + trospium) by Karuna Therapeutics.[5] Trospium is a non-CNS penetrant non-selective muscarinic antagonist to quell peripheral muscarinic agonist-dependent side effects. Xanomeline's mechanism of action is hypothesized to be via rebalancing key neurotransmitter circuits, including acetylcholine, dopamine, and glutamate, which are disrupted in schizophrenia and related diseases.[6]
Chemistry
Xanomeline has structural and pharmacological similarities to the main psychoactive ingredient in betel (areca) nut, arecoline, and the natural muscarinic receptor neurotransmitter, acetylcholine.[1][2] Xanomeline is an achiral and lipophilic small molecule with a molecular weight of 281.4 (also known as hexyloxy-TZTP, LY246708, Lumeron, Memcor - Eli Lilly; NNC 11-0232 - Novo Nordisk; Kar-XT, Karuna Therapeutics). Xanomeline's physical chemical properties, including low molecular weight, lipophilicity, and absence of hydrogen bond donors, favor its entry into the brain with a high brain to plasma ratio (> 10:1).[3]
Pharmacology
Xanomeline is an agonist that primarily targets the muscarinic acetylcholine receptor family of five muscarinic receptor subtypes, which are designated M1-M5.[1] While it binds with near identical affinity to all five of the muscarinic receptor subtypes as measured by displacement of a muscarinic radioligand, the preponderance of evidence suggests that xanomeline acts preferentially in the central nervous system as a functionally selective partial agonist at the M1 and M4 muscarinic receptors. It has more modest partial agonist pharmacology at the M2, M3 and M5 receptors.[7][8]
Central nervous system
Xanomeline regulates key dopaminergic and glutamatergic circuits in the brain that are thought to be imbalanced in patients suffering from neuropsychiatric and neurological diseases such as schizophrenia and Alzheimer's disease through stimulation primarily of central M1 and M4 muscarinic receptor subtypes. Muscarinic M1 and M4 receptors have been shown in preclinical studies to be expressed in areas important for dopamine and glutamate neural circuit regulation (e.g. frontal cortex and dorsal and ventral striatum,).[6][9] Xanomeline has shown antipsychotic-like activity in various preclinical behavioral models [6] which is dependent on M1 and M4 receptor activation.[10]
Clinical development
Xanomeline was first discovered in a therapeutic development collaboration between Eli Lilly & Co. and Novo Nordisk pharmaceutical companies in the early 1990s.[2][3] Eli Lilly led the first clinical development effort of xanomeline through a phase 2 clinical trial to test the hypothesis that it would improve cognition in patients suffering from cognitive decline observed in Alzheimer's disease [11] and later in a small placebo-controlled study in schizophrenia. Xanomeline's development was discontinued primarily due to cholinergic side effects observed in clinical studies.[12] Further development was enabled through a novel co-formulation strategy with the peripherally restricted muscarinic antagonist, trospium, to quell the peripheral cholinergic side effects.[5] This new dual drug formulation is currently called KarXT.
See also
References
- ↑ 1.0 1.1 1.2 "Novel functional M1 selective muscarinic agonists. Synthesis and structure-activity relationships of 3-(1,2,5-thiadiazolyl)-1,2,5,6-tetrahydro-1-methylpyridines". Journal of Medicinal Chemistry 35 (12): 2274–2283. June 1992. doi:10.1021/jm00090a019. PMID 1613751.
- ↑ 2.0 2.1 2.2 "Classics in Chemical Neuroscience: Xanomeline". ACS Chemical Neuroscience 8 (3): 435–443. March 2017. doi:10.1021/acschemneuro.7b00001. PMID 28141924.
- ↑ 3.0 3.1 3.2 "Xanomeline: a selective muscarinic agonist for the treatment of Alzheimer's disease". Drug Development Research 40 (2): 158–170. 1997. doi:10.1002/(SICI)1098-2299(199702)40:2<158::AID-DDR6>3.0.CO;2-K.
- ↑ "Xanomeline, an M(1)/M(4) preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice". Schizophrenia Research 42 (3): 249–259. May 2000. doi:10.1016/s0920-9964(99)00138-3. PMID 10785583.
- ↑ 5.0 5.1 "Muscarinic Cholinergic Receptor Agonist and Peripheral Antagonist for Schizophrenia". The New England Journal of Medicine 384 (8): 717–726. February 2021. doi:10.1056/NEJMoa2017015. PMID 33626254.
- ↑ 6.0 6.1 6.2 "Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists". CNS Drug Reviews 9 (2): 159–186. 2003. doi:10.1111/j.1527-3458.2003.tb00247.x. PMID 12847557.
- ↑ "Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists". European Journal of Pharmacology 605 (1–3): 53–56. March 2009. doi:10.1016/j.ejphar.2008.12.044. PMID 19168056.
- ↑ "Striatal, Hippocampal, and Cortical Networks Are Differentially Responsive to the M4- and M1-Muscarinic Acetylcholine Receptor Mediated Effects of Xanomeline". ACS Chemical Neuroscience 10 (3): 1753–1764. March 2019. doi:10.1021/acschemneuro.8b00625. PMID 30480428.
- ↑ "Positive allosteric modulation of M1 and M4 muscarinic receptors as potential therapeutic treatments for schizophrenia". Neuropharmacology 136 (Pt C): 438–448. July 2018. doi:10.1016/j.neuropharm.2017.09.012. PMID 28893562.
- ↑ "Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice". European Journal of Pharmacology 603 (1–3): 147–149. January 2009. doi:10.1016/j.ejphar.2008.12.020. PMID 19111716.
- ↑ "Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease". Archives of Neurology 54 (4): 465–473. April 1997. doi:10.1001/archneur.1997.00550160091022. PMID 9109749.
- ↑ "Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia". The American Journal of Psychiatry 165 (8): 1033–1039. August 2008. doi:10.1176/appi.ajp.2008.06091591. PMID 18593778.
Further reading
- "Towards a muscarinic hypothesis of schizophrenia". Molecular Psychiatry 12 (3): 232–246. March 2007. doi:10.1038/sj.mp.4001924. PMID 17146471.
- "M1-M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system". Receptors & Channels 9 (4): 279–290. 1 January 2003. doi:10.3109/10606820308262. PMID 12893539.
- "Muscarinic Acetylcholine Receptor Agonists as Novel Treatments for Schizophrenia". The American Journal of Psychiatry 179 (9): 611–627. September 2022. doi:10.1176/appi.ajp.21101083. PMID 35758639.
 |

