Chemistry:Tacrine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cognex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693039 |
| Pregnancy category |
|
| Routes of administration | Oral, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 2.4–36% (oral) |
| Protein binding | 55% |
| Metabolism | Hepatic (CYP1A2) |
| Elimination half-life | 2–4 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
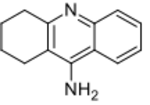
| Formula | C13H14N2 |
| Molar mass | 198.269 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 183 °C (361 °F) |
| Boiling point | 358 °C (676 °F) |
| |
| |
| (verify) | |
Tacrine is a centrally acting acetylcholinesterase inhibitor and indirect cholinergic agonist (parasympathomimetic). It was the first centrally acting cholinesterase inhibitor approved for the treatment of Alzheimer's disease, and was marketed under the trade name Cognex. Tacrine was first synthesised by Adrien Albert at the University of Sydney in 1949. It also acts as a histamine N-methyltransferase inhibitor.[1]
Clinical use
Tacrine was the prototypical cholinesterase inhibitor for the treatment of Alzheimer's disease. William K. Summers received a patent for this use in 1989.[2][3][4] Studies found that it may have a small beneficial effect on cognition and other clinical measures, though study data was limited and the clinical relevance of these findings was unclear.[5][6]
Tacrine has been discontinued in the United States of America [7] in 2013, due to concerns over safety.[8]
Tacrine was also described as an analeptic agent used to promote mental alertness.[9]
Adverse Effects
- Very common (>10% incidence) adverse effects include[7]
- Increased liver function tests (LFT)
- Nausea
- Vomiting
- Diarrhea
- Headache
- Dizziness
- Indigestion
- Belching
- Abdominal pain
- Myalgia — muscle pain
- Confusion
- Ataxia — decreased control over bodily movements.
- Insomnia
- Rhinitis
- Rash
- Fatigue
- Weight loss
- Constipation
- Somnolence
- Tremor
- Anxiety
- Urinary incontinence
- Hallucinations
- Agitation
- Conjunctivitis (a link to tacrine treatment has not been conclusively proven)
- Diaphoresis — sweating.
- Uncommon/rare (<1% incidence) adverse effects include[10]
- Hepatotoxicity (that is toxic effects on the liver)
- Ototoxicity (hearing/ear damage; a link to tacrine treatment has not been conclusively proven)
- Seizures
- Agranulocytosis (a link between treatment and this adverse effect has not been proven) — a potentially fatal drop in white blood cells, the body's immune/defensive cells.
- Taste changes
- Unknown incidence adverse effects include[10]
- Urinary tract infection
- Delirium
- Other optic effects such as glaucoma, cataracts, etc. (also not conclusively linked to tacrine treatment)
- Depression
- Suicidal ideation and behaviour
- Hypotension
- Bradycardia
Overdose
As stated above, overdosage of tacrine may give rise to severe side effects such as nausea, vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Atropine is a popular treatment for overdose.[10]
Pharmacokinetics
Major form of metabolism is in the liver via hydroxylation of benzylic carbon by CYP1A2. This forms the major metabolite 1-hydroxy-tacrine (velnacrine) which is still active.[10]
References
- ↑ "Actions of tacrine and galanthamine on histamine-N-methyltransferase". Methods and Findings in Experimental and Clinical Pharmacology 27 (3): 161–165. April 2005. doi:10.1358/mf.2005.27.3.890872. PMID 15834447.
- ↑ Summers WK, "Administration of monoamine acridines in cholinergic neuronal deficit states", US patent 4816456, issued 28 March 1989
- ↑ "A Psychiatrist's work leads to a US study of Alzheimer's drug: but Dr. Summers shuns test, seeks to widen his own; is Memory really aided; Fee-for research Furor". Wall Street Journal: A-1. 4 August 1987.
- ↑ "New Mexico Doctor invents drugs, supplements for Alzheimer's disease, Multiple Sclerosis.". NM Bus Weekly. 25 March 2005. https://www.bizjournals.com/albuquerque/stories/2005/03/28/smallb2.html.
- ↑ "Cholinesterase inhibition for Alzheimer disease: a meta-analysis of the tacrine trials. Dementia Trialists' Collaboration". JAMA 280 (20): 1777–1782. November 1998. doi:10.1001/jama.280.20.1777. PMID 9842955.
- ↑ Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. 2003. ISBN 978-0-443-07145-4..
- ↑ 7.0 7.1 7.2 "tacrine (Discontinued) - Cognex". Medscape Reference. WebMD. http://reference.medscape.com/drug/tacrine-343070.
- ↑ "Tacrine". LiverTox. U.S. National Institutes of Health. http://www.livertox.nih.gov/Tacrine.htm.
- ↑ Dictionary of Drugs. 1990. doi:10.1007/978-1-4757-2085-3. ISBN 978-1-4757-2087-7.
- ↑ 10.0 10.1 10.2 10.3 10.4 Truven Health Analytics, Inc. DRUGDEX® System (Internet) [cited 2013 Oct 8]. Greenwood Village, CO: Thomsen Healthcare; 2013.
External links
- Acetylcholinesterase: A gorge-ous enzyme QUite Interesting PDB Structure article at PDBe
 |
