Chemistry:Tin(II) bromide
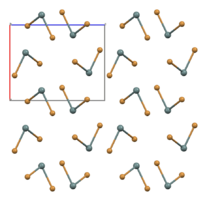
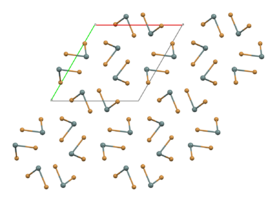
part of an (SnBr2)∞ chain in the solid state[1]
| |
| Names | |
|---|---|
| Other names
tin dibromide, stannous bromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| SnBr2 | |
| Molar mass | 278.518 g/mol |
| Appearance | yellow powder |
| Density | 5.12 g/cm3, solid |
| Melting point | 215 °C (419 °F; 488 K) |
| Boiling point | 639 °C (1,182 °F; 912 K) |
| Structure | |
| related to PbCl2 | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.[3]
Structure and bonding
In the gas phase SnBr2 is non-linear with a bent configuration similar to SnCl2 in the gas phase. The Br-Sn-Br angle is 95° and the Sn-Br bond length is 255pm.[4] There is evidence of dimerisation in the gaseous phase.[5] The solid state structure is related to that of SnCl2 and PbCl2 and the tin atoms have five near bromine atom neighbours in an approximately trigonal bipyramidal configuration.[6] Two polymorphs exist: a room-temperature orthorhombic polymorph, and a high-temperature hexagonal polymorph. Both contain (SnBr2)∞ chains but the packing arrangement differs.[1]
Preparation
Tin(II) bromide can be prepared by the reaction of metallic tin and HBr distilling off the H2O/HBr and cooling:[9]
- Sn + 2 HBr → SnBr2 + H2
However, the reaction will produce tin (IV) bromide in the presence of oxygen.
Reactions
SnBr2 is soluble in donor solvents such as acetone, pyridine and dimethylsulfoxide to give pyramidal adducts.[9]
A number of hydrates are known, 2SnBr2·H2O, 3SnBr2·H2O & 6SnBr2·5H2O which in the solid phase have tin coordinated by a distorted trigonal prism of 6 bromine atoms with Br or H2O capping 1 or 2 faces.[3]
When dissolved in HBr the pyramidal SnBr3− ion is formed.[3]
Like SnCl2 it is a reducing agent. With a variety of alkyl bromides oxidative addition can occur to yield the alkyltin tribromide[10] e.g.
- SnBr2 + RBr → RSnBr3
Tin(II) bromide can act as a Lewis acid forming adducts with donor molecules e.g. trimethylamine where it forms NMe3·SnBr2 and 2NMe3·SnBr2 [11] It can also act as both donor and acceptor in, for example, the complex F3B·SnBr2·NMe3 where it is a donor to boron trifluoride and an acceptor to trimethylamine.[12]
References
- ↑ 1.0 1.1 1.2 1.3 Eckold, Pierre; Hügel, Werner; Dinnebier, Robert E.; Niewa, Rainer (2005). "Two Modifications of Tin(II) Bromide". Z. Anorg. Allg. Chem. 641 (8-9): 1467-1472. doi:10.1002/zaac.201500108.
- ↑ "Tin(II) bromide" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/66224#section=Safety-and-Hazards.
- ↑ 3.0 3.1 3.2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ J.L Wardell "Tin:Inorganic Chemistry" Encyclopedia of Inorganic Chemistry Ed: R Bruce King John Wiley & Sons (1994) ISBN:0-471-93620-0
- ↑ K. Hilpert; M. Miller; F. Ramondo (1991). "Thermochemistry of tetrabromoditin and bromoiodotin gaseous". J. Phys. Chem. 95 (19): 7261–7266. doi:10.1021/j100172a031.
- ↑ Abrahams I.; Demetriou D.Z. (2000). "Inert Pair Effects in Tin and Lead Dihalides: Crystal Structure of Tin(II) Bromide". Journal of Solid State Chemistry 149 (1): 28–32. doi:10.1006/jssc.1999.8489. Bibcode: 2000JSSCh.149...28A.
- ↑ ICSD Entry: 429132. Cambridge Crystallographic Data Centre. https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=1737946&DatabaseToSearch=Published. Retrieved 2022-02-09.
- ↑ ICSD Entry: 429133. Cambridge Crystallographic Data Centre. https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=1737947&DatabaseToSearch=Published. Retrieved 2022-02-09.
- ↑ 9.0 9.1 Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ↑ Bulten E.J. (1975). "A convenient synthesis of (C1-C18) alkyltin tribromides". Journal of Organometallic Chemistry 97 (1): 167–172. doi:10.1016/S0022-328X(00)89463-2.
- ↑ Chung Chun Hsu; R. A. Geanangel (1977). "Synthesis and studies of trimethylamine adducts with tin(II) halides". Inorg. Chem. 16 (1): 2529–2534. doi:10.1021/ic50176a022.
- ↑ Chung Chun Hsu; R. A. Geanangel (1980). "Donor and acceptor behavior of divalent tin compounds". Inorg. Chem. 19 (1): 110–119. doi:10.1021/ic50203a024.
 |