Biology:Hydroxymethylglutaryl-CoA synthase
| 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 (soluble) | |
|---|---|
| Identifiers | |
| Symbol | HMGCS1 |
| Alt. symbols | HMGCS |
| NCBI gene | 3157 |
| HGNC | 5007 |
| OMIM | 142940 |
| RefSeq | NM_002130 |
| UniProt | Q01581 |
| Other data | |
| EC number | 2.3.3.10 |
| Locus | Chr. 5 p14-p13 |
| 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 2 (mitochondrial) | |
|---|---|
| Identifiers | |
| Symbol | HMGCS2 |
| NCBI gene | 3158 |
| HGNC | 5008 |
| OMIM | 600234 |
| RefSeq | NM_005518 |
| UniProt | P54868 |
| Other data | |
| Locus | Chr. 1 p13-p12 |
| Hydroxymethylglutaryl-coenzyme A synthase N terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 staphylococcus aureus 3-hydroxy-3-methylglutaryl-coa synthase | |||||||||
| Identifiers | |||||||||
| Symbol | HMG_CoA_synt_N | ||||||||
| Pfam | PF01154 | ||||||||
| Pfam clan | CL0046 | ||||||||
| InterPro | IPR013528 | ||||||||
| PROSITE | PDOC00942 | ||||||||
| |||||||||
| Hydroxymethylglutaryl-coenzyme A synthase C terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 staphylococcus aureus 3-hydroxy-3-methylglutaryl-coa synthase | |||||||||
| Identifiers | |||||||||
| Symbol | HMG_CoA_synt_C | ||||||||
| Pfam | PF08540 | ||||||||
| Pfam clan | CL0046 | ||||||||
| InterPro | IPR013746 | ||||||||
| PROSITE | PDOC00942 | ||||||||
| |||||||||
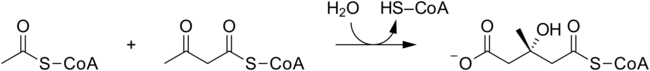
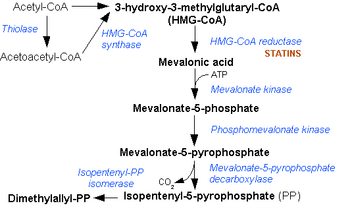
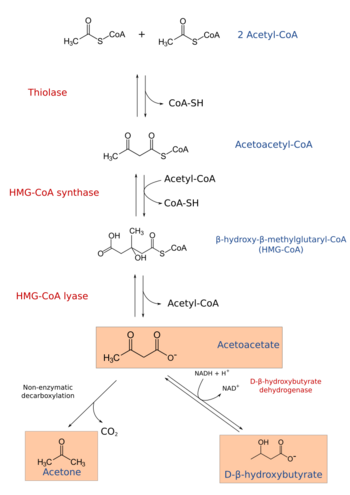
In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.
The 3 substrates of this enzyme are acetyl-CoA, H2O, and acetoacetyl-CoA, whereas its two products are (S)-3-hydroxy-3-methylglutaryl-CoA and CoA.
In humans, the protein is encoded by the HMGCS1 gene on chromosome 5.
Classification
This enzyme belongs to the family of transferases, specifically those acyltransferases that convert acyl groups into alkyl groups on transfer.
Nomenclature
The systematic name of this enzyme class is acetyl-CoA:acetoacetyl-CoA C-acetyltransferase (thioester-hydrolysing, carboxymethyl-forming). Other names in common use include (S)-3-hydroxy-3-methylglutaryl-CoA acetoacetyl-CoA-lyase, (CoA-acetylating), 3-hydroxy-3-methylglutaryl CoA synthetase, 3-hydroxy-3-methylglutaryl coenzyme A synthase, 3-hydroxy-3-methylglutaryl coenzyme A synthetase, 3-hydroxy-3-methylglutaryl-CoA synthase, 3-hydroxy-3-methylglutaryl-coenzyme A synthase, beta-hydroxy-beta-methylglutaryl-CoA synthase, HMG-CoA synthase, acetoacetyl coenzyme A transacetase, hydroxymethylglutaryl coenzyme A synthase, and hydroxymethylglutaryl coenzyme A-condensing enzyme.
Mechanism
HMG-CoA synthase contains an important catalytic cysteine residue that acts as a nucleophile in the first step of the reaction: the acetylation of the enzyme by acetyl-CoA (its first substrate) to produce an acetyl-enzyme thioester, releasing the reduced coenzyme A. The subsequent nucleophilic attack on acetoacetyl-CoA (its second substrate) leads to the formation of HMG-CoA.[1]
Biological role
This enzyme participates in 3 metabolic pathways: synthesis and degradation of ketone bodies, valine, leucine and isoleucine degradation, and butanoate metabolism.
Species distribution
HMG-CoA synthase occurs in eukaryotes, archaea, and certain bacteria.[2]
Eukaryotes
In vertebrates, there are two different isozymes of the enzyme (cytosolic and mitochondrial); in humans the cytosolic form has only 60.6% amino acid identity with the mitochondrial form of the enzyme. HMG-CoA is also found in other eukaryotes such as insects, plants, and fungi.[3]
Cytosolic
The cytosolic form is the starting point of the mevalonate pathway, which leads to cholesterol and other sterolic and isoprenoid compounds.
Mitochondrial
The mitochondrial form is responsible for the biosynthesis of ketone bodies. The gene for the mitochondrial form of the enzyme has three sterol regulatory elements in the 5' flanking region.[4] These elements are responsible for decreased transcription of the message responsible for enzyme synthesis when dietary cholesterol is high in animals: the same is observed for 3-hydroxy-3-methylglutaryl-CoA and the low density lipoprotein receptor.
Bacteria
In bacteria, isoprenoid precursors are generally synthesised via an alternative, non-mevalonate pathway, however a number of Gram-positive pathogens utilise a mevalonate pathway involving HMG-CoA synthase that is parallel to that found in eukaryotes.[5][6]
Structural studies
As of late 2007, 4 structures have been solved for this class of enzymes, with PDB accession codes 1XPK, 1XPL, 1XPM, and 2P8U.
External links
- HMG-CoA+synthase at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- ↑ "3-hydroxy-3-methylglutaryl-CoA synthase intermediate complex observed in "real-time"". Proc. Natl. Acad. Sci. U.S.A. 101 (47): 16442–7. November 2004. doi:10.1073/pnas.0405809101. PMID 15498869.
- ↑ Bahnson BJ (November 2004). "An atomic-resolution mechanism of 3-hydroxy-3-methylglutaryl-CoA synthase". Proc. Natl. Acad. Sci. U.S.A. 101 (47): 16399–400. doi:10.1073/pnas.0407418101. PMID 15546978. Bibcode: 2004PNAS..10116399B.
- ↑ "Isolation, endocrine regulation and mRNA distribution of the 3-hydroxy-3-methylglutaryl coenzyme A synthase (HMG-S) gene from the pine engraver, Ips pini (Coleoptera: Scolytidae)". Insect Molecular Biology 15 (2): 187–95. April 2006. doi:10.1111/j.1365-2583.2006.00627.x. PMID 16640729.
- ↑ Goldstein J.L., Brown M.S. (1990) Regulation of the mevalonate pathway. Nature 343, 425-430
- ↑ "A structural limitation on enzyme activity: the case of HMG-CoA synthase". Biochemistry 45 (48): 14407–14. December 2006. doi:10.1021/bi061505q. PMID 17128980.
- ↑ "X-ray crystal structures of HMG-CoA synthase from Enterococcus faecalis and a complex with its second substrate/inhibitor acetoacetyl-CoA". Biochemistry 44 (43): 14256–67. November 2005. doi:10.1021/bi051487x. PMID 16245942.
- RUDNEY H (1957). "The biosynthesis of beta-hydroxy-beta-methylglutaric acid". J. Biol. Chem. 227 (1): 363–77. doi:10.1016/S0021-9258(18)70822-3. PMID 13449080.
 |