Chemistry:Timeline of chemistry

This timeline of chemistry lists important works, discoveries, ideas, inventions, and experiments that significantly changed humanity's understanding of the modern science known as chemistry, defined as the scientific study of the composition of matter and of its interactions.
Known as "the central science", the study of chemistry is strongly influenced by, and exerts a strong influence on, many other scientific and technological fields. Many historical developments that are considered to have had a significant impact upon our modern understanding of chemistry are also considered to have been key discoveries in such fields as physics, biology, astronomy, geology, and materials science.[1]
Pre-17th century



Prior to the acceptance of the scientific method and its application to the field of chemistry, it is somewhat controversial to consider many of the people listed below as "chemists" in the modern sense of the word. However, the ideas of certain great thinkers, either for their prescience, or for their wide and long-term acceptance, bear listing here.
c. 450 BC
Empedocles asserts that all things are composed of four primal roots (later to be renamed stoicheia or elements): earth, air, fire, and water, whereby two active and opposing cosmic forces, love and strife, act upon these elements, combining and separating them into infinitely varied forms.[2]
c. 440 BC
Leucippus and Democritus propose the idea of the atom, an indivisible particle that all matter is made of. This idea is largely rejected by natural philosophers in favor of the Aristotelian view (see below).[3][4]
c. 360 BC
Plato coins term ‘elements’ (stoicheia) and in his dialogue Timaeus, which includes a discussion of the composition of inorganic and organic bodies and is a rudimentary treatise on chemistry, assumes that the minute particle of each element had a special geometric shape: tetrahedron (fire), octahedron (air), icosahedron (water), and cube (earth).[5]
c. 350 BC
Aristotle, expanding on Empedocles, proposes idea of a substance as a combination of matter and form. Describes theory of the Five Elements, fire, water, earth, air, and aether. This theory is largely accepted throughout the western world for over 1000 years.[6]
c. 50 BC
Lucretius publishes De Rerum Natura, a poetic description of the ideas of atomism.[7]
c. 300
Zosimos of Panopolis writes some of the oldest known books on alchemy, which he defines as the study of the composition of waters, movement, growth, embodying and disembodying, drawing the spirits from bodies and bonding the spirits within bodies.[8]
c. 800
The Secret of Creation (Arabic: Sirr al-khalīqa), an anonymous encyclopedic work on natural philosophy falsely attributed to Apollonius of Tyana, records the earliest known version of the long-held theory that all metals are composed of various proportions of sulfur and mercury.[9] This same work also contains the earliest known version of the Emerald Tablet,[10] a compact and cryptic Hermetic text which was still commented upon by Isaac Newton.[11]
c. 850–900
Arabic works attributed to Jābir ibn Ḥayyān (Latin: Geber) introduce a systematic classification of chemical substances, and provide instructions for deriving an inorganic compound (sal ammoniac or ammonium chloride) from organic substances (such as plants, blood, and hair) by chemical means.[12]
c. 900
Abū Bakr al-Rāzī (Latin: Rhazes), a Persian alchemist, conducts experiments with the distillation of sal ammoniac (ammonium chloride), vitriols (hydrated sulfates of various metals), and other salts,[13] representing the first step in a long process that would eventually lead to the thirteenth-century discovery of the mineral acids.[14]
c. 1000
Abū al-Rayhān al-Bīrūnī[15] and Avicenna,[16] both Persian philosophers, deny the possibility of the transmutation of metals.
c. 1100–1200
Recipes for the production of aqua ardens ("burning water", i.e., ethanol) by distilling wine with common salt start to appear in a number of Latin alchemical works.[17]
c. 1220
Robert Grosseteste publishes several Aristotelian commentaries where he lays out an early framework for the scientific method.[18]
c. 1250
The works of Taddeo Alderotti (1223–1296) describe a method for concentrating ethanol involving repeated fractional distillation through a water-cooled still, by which an ethanol purity of 90% could be obtained.[19]
c. 1260
St Albertus Magnus discovers arsenic[20] and silver nitrate.[21]{{Better source needed|date=January 2021} to sulfuric acid.[22]
c. 1267
Roger Bacon publishes Opus Maius, which among other things, proposes an early form of the scientific method, and contains results of his experiments with gunpowder.[23]
c. 1310
Pseudo-Geber, an anonymous alchemist who wrote under the name of Geber (i.e., Jābir ibn Hayyān, see above), publishes the Summa perfectionis magisterii. This work contains experimental demonstrations of the corpuscular nature of matter that would still be used by seventeenth-century chemists such as Daniel Sennert.[24] Pseudo-Geber is one of the first alchemists to describe mineral acids such as aqua fortis or 'strong water' (nitric acid, capable of dissolving silver) and aqua regia or 'royal water' (a mixture of nitric acid and hydrochloric acid, capable of dissolving gold and platinum).Cite error: Invalid <ref> tag; refs with no name must have content
c. 1530
Paracelsus develops the study of iatrochemistry, a subdiscipline of alchemy dedicated to extending life, thus being the roots of the modern pharmaceutical industry. It is also claimed that he is the first to use the word "chemistry".[8]
1597
Andreas Libavius publishes Alchemia, a prototype chemistry textbook.[25]
17th and 18th centuries
1605
Sir Francis Bacon publishes The Proficience and Advancement of Learning, which contains a description of what would later be known as the scientific method.[26]
1605
Michal Sedziwój publishes the alchemical treatise A New Light of Alchemy which proposed the existence of the "food of life" within air, much later recognized as oxygen.[27]
1615
Jean Beguin publishes the Tyrocinium Chymicum, an early chemistry textbook, and in it draws the first-ever chemical equation.[28]
1637
René Descartes publishes Discours de la méthode, which contains an outline of the scientific method.[29]
1648
Posthumous publication of the book Ortus medicinae by Jan Baptist van Helmont, which is cited by some as a major transitional work between alchemy and chemistry, and as an important influence on Robert Boyle. The book contains the results of numerous experiments and establishes an early version of the law of conservation of mass.[30]

1661
Robert Boyle publishes The Sceptical Chymist, a treatise on the distinction between chemistry and alchemy. It contains some of the earliest modern ideas of atoms, molecules, and chemical reaction, and marks the beginning of the history of modern chemistry.[31]
1662
Robert Boyle proposes Boyle's law, an experimentally based description of the behavior of gases, specifically the relationship between pressure and volume.[31]
1735
Swedish chemist Georg Brandt analyzes a dark blue pigment found in copper ore. Brandt demonstrated that the pigment contained a new element, later named cobalt.[32][33]
1754
Joseph Black isolates carbon dioxide, which he called "fixed air".[34]

1757
Louis Claude Cadet de Gassicourt, while investigating arsenic compounds, creates Cadet's fuming liquid, later discovered to be cacodyl oxide, considered to be the first synthetic organometallic compound.[35]
1758
Joseph Black formulates the concept of latent heat to explain the thermochemistry of phase changes.[36]
1766
Henry Cavendish discovers hydrogen as a colorless, odourless gas that burns and can form an explosive mixture with air.[37]
1773–1774
Carl Wilhelm Scheele and Joseph Priestley independently isolate oxygen, called by Priestley "dephlogisticated air" and Scheele "fire air".[38][39]

1778
Antoine Lavoisier, considered "The father of modern chemistry",[40] recognizes and names oxygen, and recognizes its importance and role in combustion.[41]
1787
Antoine Lavoisier publishes Méthode de nomenclature chimique, the first modern system of chemical nomenclature.[41]
1787
Jacques Charles proposes Charles's law, a corollary of Boyle's law, describes relationship between temperature and volume of a gas.[42]
1789
Antoine Lavoisier publishes Traité Élémentaire de Chimie, the first modern chemistry textbook. It is a complete survey of (at that time) modern chemistry, including the first concise definition of the law of conservation of mass, and thus also represents the founding of the discipline of stoichiometry or quantitative chemical analysis.[41][43]
1797
Joseph Proust proposes the law of definite proportions, which states that elements always combine in small, whole number ratios to form compounds.[44]
1800
Alessandro Volta devises the first chemical battery, thereby founding the discipline of electrochemistry.[45]
19th century

1803
John Dalton proposes Dalton's law, which describes relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture.[46]
1805
Joseph Louis Gay-Lussac discovers that water is composed of two parts hydrogen and one part oxygen by volume.[47]
1808
Joseph Louis Gay-Lussac collects and discovers several chemical and physical properties of air and of other gases, including experimental proofs of Boyle's and Charles's laws, and of relationships between density and composition of gases.[48]
1808
John Dalton publishes New System of Chemical Philosophy, which contains first modern scientific description of the atomic theory, and clear description of the law of multiple proportions.[46]
1808
Jöns Jakob Berzelius publishes Lärbok i Kemien in which he proposes modern chemical symbols and notation, and of the concept of relative atomic weight.[49]
1811
Amedeo Avogadro proposes Avogadro's law, that equal volumes of gases under constant temperature and pressure contain equal number of molecules.[50]
1825
Friedrich Wöhler and Justus von Liebig perform the first confirmed discovery and explanation of isomers, earlier named by Berzelius. Working with cyanic acid and fulminic acid, they correctly deduce that isomerism was caused by differing arrangements of atoms within a molecular structure.[51]
1827
William Prout classifies biomolecules into their modern groupings: carbohydrates, proteins and lipids.[52]
1828
Friedrich Wöhler synthesizes urea, thereby establishing that organic compounds could be produced from inorganic starting materials, disproving the theory of vitalism.[51]
1832
Friedrich Wöhler and Justus von Liebig discover and explain functional groups and radicals in relation to organic chemistry.[51]
1840
Germain Hess proposes Hess's law, an early statement of the law of conservation of energy, which establishes that energy changes in a chemical process depend only on the states of the starting and product materials and not on the specific pathway taken between the two states.[53]
1847
Hermann Kolbe obtains acetic acid from completely inorganic sources, further disproving vitalism.[54]
1848
Lord Kelvin establishes concept of absolute zero, the temperature at which all molecular motion ceases.[55]
1849
Louis Pasteur discovers that the racemic form of tartaric acid is a mixture of the levorotatory and dextrotatory forms, thus clarifying the nature of optical rotation and advancing the field of stereochemistry.[56]
1852
August Beer proposes Beer's law, which explains the relationship between the composition of a mixture and the amount of light it will absorb. Based partly on earlier work by Pierre Bouguer and Johann Heinrich Lambert, it establishes the analytical technique known as spectrophotometry.[57]
1855
Benjamin Silliman, Jr. pioneers methods of petroleum cracking, which makes the entire modern petrochemical industry possible.[58]
1856
William Henry Perkin synthesizes Perkin's mauve, the first synthetic dye. Created as an accidental byproduct of an attempt to create quinine from coal tar. This discovery is the foundation of the dye synthesis industry, one of the earliest successful chemical industries.[59]
1857
Friedrich August Kekulé von Stradonitz proposes that carbon is tetravalent, or forms exactly four chemical bonds.[60]
1859–1860
Gustav Kirchhoff and Robert Bunsen lay the foundations of spectroscopy as a means of chemical analysis, which lead them to the discovery of caesium and rubidium. Other workers soon used the same technique to discover indium, thallium, and helium.[61]
1860
Stanislao Cannizzaro, resurrecting Avogadro's ideas regarding diatomic molecules, compiles a table of atomic weights and presents it at the 1860 Karlsruhe Congress, ending decades of conflicting atomic weights and molecular formulas, and leading to Mendeleev's discovery of the periodic law.[62]
1862
Alexander Parkes exhibits Parkesine, one of the earliest synthetic polymers, at the International Exhibition in London. This discovery formed the foundation of the modern plastics industry.[63]
1862
Alexandre-Emile Béguyer de Chancourtois publishes the telluric helix, an early, three-dimensional version of the periodic table of the elements.[64]
1864
John Newlands proposes the law of octaves, a precursor to the periodic law.[64]
1864
Lothar Meyer develops an early version of the periodic table, with 28 elements organized by valence.[65]
1864
Cato Maximilian Guldberg and Peter Waage, building on Claude Louis Berthollet's ideas, proposed the law of mass action.[66][67][68]
1865
Johann Josef Loschmidt determines exact number of molecules in a mole, later named Avogadro constant.[69]
1865
Friedrich August Kekulé von Stradonitz, based partially on the work of Loschmidt and others, establishes structure of benzene as a six carbon ring with alternating single and double bonds.[60]
1865
Adolf von Baeyer begins work on indigo dye, a milestone in modern industrial organic chemistry which revolutionizes the dye industry.[70]
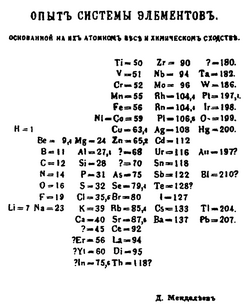
1869
Dmitri Mendeleev publishes the first modern periodic table, with the 66 known elements organized by atomic weights. The strength of his table was its ability to accurately predict the properties of as-yet unknown elements.[64][65]
1873
Jacobus Henricus van 't Hoff and Joseph Achille Le Bel, working independently, develop a model of chemical bonding that explains the chirality experiments of Pasteur and provides a physical cause for optical activity in chiral compounds.[71]
1876
Josiah Willard Gibbs publishes On the Equilibrium of Heterogeneous Substances, a compilation of his work on thermodynamics and physical chemistry which lays out the concept of free energy to explain the physical basis of chemical equilibria.[72]
1877
Ludwig Boltzmann establishes statistical derivations of many important physical and chemical concepts, including entropy, and distributions of molecular velocities in the gas phase.[73]
1883
Svante Arrhenius develops ion theory to explain conductivity in electrolytes.[74]
1884
Jacobus Henricus van 't Hoff publishes Études de Dynamique chimique, a seminal study on chemical kinetics.[75]
1884
Hermann Emil Fischer proposes structure of purine, a key structure in many biomolecules, which he later synthesized in 1898. Also begins work on the chemistry of glucose and related sugars.[76]
1884
Henry Louis Le Chatelier develops Le Chatelier's principle, which explains the response of dynamic chemical equilibria to external stresses.[77]
1885
Eugen Goldstein names the cathode ray, later discovered to be composed of electrons, and the canal ray, later discovered to be positive hydrogen ions that had been stripped of their electrons in a cathode ray tube. These would later be named protons.[78]
1893
Alfred Werner discovers the octahedral structure of cobalt complexes, thus establishing the field of coordination chemistry.[79]
1894–1898
William Ramsay discovers the noble gases, which fill a large and unexpected gap in the periodic table and led to models of chemical bonding.[80]
1897
J. J. Thomson discovers the electron using the cathode ray tube.[81]
1898
Wilhelm Wien demonstrates that canal rays (streams of positive ions) can be deflected by magnetic fields, and that the amount of deflection is proportional to the mass-to-charge ratio. This discovery would lead to the analytical technique known as mass spectrometry.[82]
1898
Maria Sklodowska-Curie and Pierre Curie isolate radium and polonium from pitchblende.[83]
c. 1900
Ernest Rutherford discovers the source of radioactivity as decaying atoms; coins terms for various types of radiation.[84]
20th century
1903
Mikhail Semyonovich Tsvet invents chromatography, an important analytic technique.[85]
1904
Hantaro Nagaoka proposes an early nuclear model of the atom, where electrons orbit a dense massive nucleus.[86]
1905
Fritz Haber and Carl Bosch develop the Haber process for making ammonia from its elements, a milestone in industrial chemistry with deep consequences in agriculture.[87]
1905
Albert Einstein explains Brownian motion in a way that definitively proves atomic theory.[88]
1907
Leo Hendrik Baekeland invents bakelite, one of the first commercially successful plastics.[89]

1909
Robert Millikan measures the charge of individual electrons with unprecedented accuracy through the oil drop experiment, confirming that all electrons have the same charge and mass.[90]
1909
S. P. L. Sørensen invents the pH concept and develops methods for measuring acidity.[91]
1911
Antonius van den Broek proposes the idea that the elements on the periodic table are more properly organized by positive nuclear charge rather than atomic weight.[92]
1911
The first Solvay Conference is held in Brussels, bringing together most of the most prominent scientists of the day. Conferences in physics and chemistry continue to be held periodically to this day.[93]
1911
Ernest Rutherford, Hans Geiger, and Ernest Marsden perform the gold foil experiment, which proves the nuclear model of the atom, with a small, dense, positive nucleus surrounded by a diffuse electron cloud.[84]
1912
William Henry Bragg and William Lawrence Bragg propose Bragg's law and establish the field of X-ray crystallography, an important tool for elucidating the crystal structure of substances.[94]
1912
Peter Debye develops the concept of molecular dipole to describe asymmetric charge distribution in some molecules.[95]

1913
Niels Bohr introduces concepts of quantum mechanics to atomic structure by proposing what is now known as the Bohr model of the atom, where electrons exist only in strictly defined orbitals.[96]
1913
Henry Moseley, working from Van den Broek's earlier idea, introduces concept of atomic number to fix inadequacies of Mendeleev's periodic table, which had been based on atomic weight.[97]
1913
Frederick Soddy proposes the concept of isotopes, that elements with the same chemical properties may have differing atomic weights.[98]
1913
J. J. Thomson expanding on the work of Wien, shows that charged subatomic particles can be separated by their mass-to-charge ratio, a technique known as mass spectrometry.[99]
1916
Gilbert N. Lewis publishes "The Atom and the Molecule", the foundation of valence bond theory.[100]
1921
Otto Stern and Walther Gerlach establish concept of quantum mechanical spin in subatomic particles.[101]
1923
Gilbert N. Lewis and Merle Randall publish Thermodynamics and the Free Energy of Chemical Substances, first modern treatise on chemical thermodynamics.[102]
1923
Gilbert N. Lewis develops the electron pair theory of acid/base reactions.[100]
1924
Louis de Broglie introduces the wave-model of atomic structure, based on the ideas of wave–particle duality.[103]
1925
Wolfgang Pauli develops the exclusion principle, which states that no two electrons around a single nucleus may have the same quantum state, as described by four quantum numbers.[104]
- 1926
- Erwin Schrödinger proposes the Schrödinger equation, which provides a mathematical basis for the wave model of atomic structure.[105]
1927
Werner Heisenberg develops the uncertainty principle which, among other things, explains the mechanics of electron motion around the nucleus.[106]
1927
Fritz London and Walter Heitler apply quantum mechanics to explain covalent bonding in the hydrogen molecule,[107] which marked the birth of quantum chemistry.[108]
1929
Linus Pauling publishes Pauling's rules, which are key principles for the use of X-ray crystallography to deduce molecular structure.[109]
1931
Erich Hückel proposes Hückel's rule, which explains when a planar ring molecule will have aromatic properties.[110]
1931
Harold Urey discovers deuterium by fractionally distilling liquid hydrogen.[111]
1932
James Chadwick discovers the neutron.[112]
1932–1934
Linus Pauling and Robert Mulliken quantify electronegativity, devising the scales that now bear their names.[113]
1935
Wallace Carothers leads a team of chemists at DuPont who invent nylon, one of the most commercially successful synthetic polymers in history.[114]
1937
Carlo Perrier and Emilio Segrè perform the first confirmed synthesis of technetium-97, the first artificially produced element, filling a gap in the periodic table. Though disputed, the element may have been synthesized as early as 1925 by Walter Noddack and others.[115]
1937
Eugene Houdry develops a method of industrial scale catalytic cracking of petroleum, leading to the development of the first modern oil refinery.[116]
1937
Pyotr Kapitsa, John Allen and Don Misener produce supercooled helium-4, the first zero-viscosity superfluid, a substance that displays quantum mechanical properties on a macroscopic scale.[117]
1939
Otto Hahn and Lise Meitner discover the process of nuclear fission in uranium.[118]
1939
Linus Pauling publishes The Nature of the Chemical Bond, a compilation of a decades worth of work on chemical bonding. It is one of the most important modern chemical texts. It explains hybridization theory, covalent bonding and ionic bonding as explained through electronegativity, and resonance as a means to explain, among other things, the structure of benzene.[109]
1940
Edwin McMillan and Philip H. Abelson identify neptunium, the lightest and first synthesized transuranium element, found in the products of uranium fission. McMillan would found a lab at Berkeley that would be involved in the discovery of many new elements and isotopes.[119]
1941
Glenn T. Seaborg takes over McMillan's work creating new atomic nuclei. Pioneers method of neutron capture and later through other nuclear reactions. Would become the principal or co-discoverer of nine new chemical elements, and dozens of new isotopes of existing elements.[119]
1944
Robert Burns Woodward and William von Eggers Doering successfully synthesized of quinine. This achievement, characterized of fully artificial chemicals as source for synthesis process, opened an era called as "Woodwardian era" or "chemical era" when many drugs and chemicals, as well as organic synthesis methods invented. Due to the growth of chemical industry, many fields has grown, such as drug industry.[120]
1945–1946
- Felix Bloch and Edward Mills Purcell develop the process of nuclear magnetic resonance, an analytical technique important in elucidating structures of molecules, especially in organic chemistry.[121]
- Jacob A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell perform the first confirmed synthesis of Promethium, filling in the last "gap" in the periodic table.[122]
1951
Linus Pauling uses X-ray crystallography to deduce the secondary structure of proteins.[109]
1952
Alan Walsh pioneers the field of atomic absorption spectroscopy, an important quantitative spectroscopy method that allows one to measure specific concentrations of a material in a mixture.[123]
1952
Robert Burns Woodward, Geoffrey Wilkinson, and Ernst Otto Fischer discover the structure of ferrocene, one of the founding discoveries of the field of organometallic chemistry.[124]
1953
James D. Watson and Francis Crick propose the structure of DNA, opening the door to the field of molecular biology.[125]
1957
Jens Skou discovers Na⁺/K⁺-ATPase, the first ion-transporting enzyme.[126]
1958
Max Perutz and John Kendrew use X-ray crystallography to elucidate a protein structure, specifically sperm whale myoglobin.[127]
1962
Neil Bartlett synthesizes xenon hexafluoroplatinate, showing for the first time that the noble gases can form chemical compounds.[128]
1962
George Olah observes carbocations via superacid reactions.[129]
1964
Richard R. Ernst performs experiments that will lead to the development of the technique of Fourier transform NMR. This would greatly increase the sensitivity of the technique, and open the door for magnetic resonance imaging or MRI.[130]
1965
Robert Burns Woodward and Roald Hoffmann propose the Woodward–Hoffmann rules, which use the symmetry of molecular orbitals to explain the stereochemistry of chemical reactions.[124]
1966
Hitoshi Nozaki and Ryōji Noyori discovered the first example of asymmetric catalysis (hydrogenation) using a structurally well-defined chiral transition metal complex.[131][132]
1970
John Pople develops the Gaussian program greatly easing computational chemistry calculations.[133]
1971
Yves Chauvin offered an explanation of the reaction mechanism of olefin metathesis reactions.[134]
1975
Karl Barry Sharpless and group discover a stereoselective oxidation reactions including Sharpless epoxidation,[135][136] Sharpless asymmetric dihydroxylation,[137][138][139] and Sharpless oxyamination.[140][141][142]
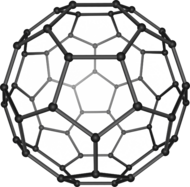
1985
Harold Kroto, Robert Curl and Richard Smalley discover fullerenes, a class of large carbon molecules superficially resembling the geodesic dome designed by architect R. Buckminster Fuller.[143]
1991
Sumio Iijima uses electron microscopy to discover a type of cylindrical fullerene known as a carbon nanotube, though earlier work had been done in the field as early as 1951. This material is an important component in the field of nanotechnology.[144]
1994
First total synthesis of Taxol by Robert A. Holton and his group.[145][146][147]
1995
Eric Cornell and Carl Wieman produce the first Bose–Einstein condensate, a substance that displays quantum mechanical properties on the macroscopic scale.[148]
See also
- History of chemistry
- Nobel Prize in chemistry
- List of Nobel laureates in Chemistry
- Timeline of chemical elements discoveries
References
- ↑ "Chemistry – The Central Science". The Chemistry Hall of Fame. York University. http://www.chem.yorku.ca/hall_of_fame/whychem.htm.
- ↑ Kingsley, K. Scarlett and Richard Parry, "Empedocles", The Stanford Encyclopedia of Philosophy (Summer 2020 Edition), Edward N. Zalta (ed.).
- ↑ Berryman, Sylvia (2004-08-14). "Leucippus". Stanford Encyclopedia of Philosophy. Metaphysics Research Lab, CSLI, Stanford University. http://plato.stanford.edu/entries/leucippus/. Retrieved 2007-03-11.
- ↑ Berryman, Sylvia (2004-08-15). "Democritus". Stanford Encyclopedia of Philosophy. Metaphysics Research Lab, CSLI, Stanford University. http://plato.stanford.edu/entries/democritus/. Retrieved 2007-03-11.
- ↑ Hillar, Marian (2004). "The Problem of the Soul in Aristotle's De anima". NASA WMAP. http://www.socinian.org/aristotles_de_anima.html.
- ↑ "HISTORY/CHRONOLOGY OF THE ELEMENTS". http://hilltop.bradley.edu/~rbg/el.html.
- ↑ Sedley, David (2004-08-04). "Lucretius". Stanford Encyclopedia of Philosophy. Metaphysics Research Lab, CSLI, Stanford University. http://plato.stanford.edu/entries/lucretius/. Retrieved 2007-03-11.
- ↑ 8.0 8.1 Strathern, Paul (2000). Mendeleyev's Dream – The Quest for the Elements. Berkley Books. ISBN 978-0-425-18467-7.
- ↑ Kraus, Paul 1942-1943. Jâbir ibn Hayyân: Contribution à l'histoire des idées scientifiques dans l'Islam. I. Le corpus des écrits jâbiriens. II. Jâbir et la science grecque. Cairo: Institut français d'archéologie orientale, vol. II, p. 1, note 1; Weisser, Ursula 1980. Das Buch über das Geheimnis der Schöpfung von Pseudo-Apollonios von Tyana. Berlin: De Gruyter, p. 199. On the dating and historical background of the Sirr al-khalīqa, see Kraus 1942−1943, vol. II, pp. 270–303; Weisser 1980, pp. 39–72. On the further history of this theory up to the eighteenth century, see Norris, John 2006. “The Mineral Exhalation Theory of Metallogenesis in Pre-Modern Mineral Science” in: Ambix, 53, pp. 43–65.
- ↑ Weisser 1980, p. 46.
- ↑ Isaac Newton. "Keynes MS. 28". The Chymistry of Isaac Newton. Ed. William R. Newman. June 2010.
- ↑ Stapleton, Henry E.; Azo, R.F.; Hidayat Husain, M. (1927). "Chemistry in Iraq and Persia in the Tenth Century A.D.". Memoirs of the Asiatic Society of Bengal VIII (6): 317–418. OCLC 706947607. http://www.southasiaarchive.com/Content/sarf.100203/231270. pp. 338–340; Kraus, Paul (1942–1943). Jâbir ibn Hayyân: Contribution à l'histoire des idées scientifiques dans l'Islam. I. Le corpus des écrits jâbiriens. II. Jâbir et la science grecque. Cairo: Institut Français d'Archéologie Orientale. ISBN 978-3-487-09115-0. OCLC 468740510. vol. II, pp. 41–42.
- ↑ Multhauf, Robert P. (1966). The Origins of Chemistry. London: Oldbourne. pp. 141-142.
- ↑ Multhauf 1966, pp. 162–163.
- ↑ Marmura, Michael E. (1965). "An Introduction to Islamic Cosmological Doctrines. Conceptions of Nature and Methods Used for Its Study by the Ikhwan Al-Safa'an, Al-Biruni, and Ibn Sina by Seyyed Hossein Nasr". Speculum 40 (4): 744–746. doi:10.2307/2851429.
- ↑ Robert Briffault (1938). The Making of Humanity, p. 196-197.
- ↑ Multhauf 1966, pp. 204–206.
- ↑
 Herbermann, Charles, ed (1913). "Robert Grosseteste". Catholic Encyclopedia. New York: Robert Appleton Company.
Herbermann, Charles, ed (1913). "Robert Grosseteste". Catholic Encyclopedia. New York: Robert Appleton Company.
- ↑ Holmyard, Eric John (1957). Alchemy. Harmondsworth: Penguin Books. ISBN 978-0-486-26298-7. pp. 51–52.
- ↑ Emsley, John (2001). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford: Oxford University Press. pp. 43, 513, 529. ISBN 978-0-19-850341-5.
- ↑ Davidson, Michael W. (2003-08-01). "Molecular Expressions: Science, Optics and You — Timeline — Albertus Magnus". The Florida State University. http://micro.magnet.fsu.edu/optics/timeline/people/magnus.html.
- ↑ Vladimir Karpenko, John A. Norris(2001), Vitriol in the history of Chemistry, Charles University
- ↑ O'Connor, J. J.; Robertson, E. F. (2003). "Roger Bacon". MacTutor. School of Mathematics and Statistics University of St Andrews, Scotland. http://www-groups.dcs.st-and.ac.uk/~history/Biographies/Bacon.html.
- ↑ Newman, William R. 1985. “New Light on the Identity of Geber” in: Sudhoffs Archiv, 69(1), pp. 76-90; Newman, William R. 2001. "Experimental Corpuscular Theory in Aristotelian Alchemy: From Geber to Sennert" in: Christoph Lüthy (ed.). Late Medieval and Early Modern Corpuscular Matter Theories. Leiden: Brill, 2001, pp. 291-329; Newman, William R. 2006. Atoms and Alchemy: Chymistry and the Experimental Origins of the Scientific Revolution. Chicago: University of Chicago Press.
- ↑ "From liquid to vapor and back: origins". Special Collections Department. University of Delaware Library. http://www.lib.udel.edu/ud/spec/exhibits/vapor/origins.htm.
- ↑ Asarnow, Herman (2005-08-08). "Sir Francis Bacon: Empiricism". An Image-Oriented Introduction to Backgrounds for English Renaissance Literature. University of Portland. http://faculty.up.edu/asarnow/eliz4.htm.
- ↑ "Sedziwój, Michal". infopoland: Poland on the Web. University at Buffalo. http://info-poland.buffalo.edu/web/sci_health/science/scientists/sedziwoj/link.shtml.
- ↑ Crosland, M.P. (1959). "The use of diagrams as chemical 'equations' in the lectures of William Cullen and Joseph Black". Annals of Science 15 (2): 75–90. doi:10.1080/00033795900200088.
- ↑
 Herbermann, Charles, ed (1913). "René Descartes". Catholic Encyclopedia. New York: Robert Appleton Company.
Herbermann, Charles, ed (1913). "René Descartes". Catholic Encyclopedia. New York: Robert Appleton Company.
- ↑ "Johann Baptista van Helmont". History of Gas Chemistry. Center for Microscale Gas Chemistry, Creighton University. 2005-09-25. http://mattson.creighton.edu/History_Gas_Chemistry/vanHelmont.html.
- ↑ 31.0 31.1 "Robert Boyle". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ Georg Brandt first showed cobalt to be a new metal in: G. Brandt (1735) "Dissertatio de semimetallis" (Dissertation on semi-metals), Acta Literaria et Scientiarum Sveciae (Journal of Swedish literature and sciences), vol. 4, pages 1–10.
See also: (1) G. Brandt (1746) "Rön och anmärkningar angäende en synnerlig färg — cobolt" (Observations and remarks concerning an extraordinary pigment — cobalt), Kongliga Svenska vetenskapsakademiens handlingar (Transactions of the Royal Swedish Academy of Science), vol.7, pages 119–130; (2) G. Brandt (1748) "Cobalti nova species examinata et descripta" (Cobalt, a new element examined and described), Acta Regiae Societatis Scientiarum Upsaliensis (Journal of the Royal Scientific Society of Uppsala), 1st series, vol. 3, pages 33–41; (3) James L. Marshall and Virginia R. Marshall (Spring 2003) "Rediscovery of the Elements: Riddarhyttan, Sweden," The Hexagon (official journal of the Alpha Chi Sigma fraternity of chemists), vol. 94, no. 1, pages 3–8. - ↑ Wang, Shijie (2006). "Cobalt—Its recovery, recycling, and application". Journal of the Minerals, Metals and Materials Society 58 (10): 47–50. doi:10.1007/s11837-006-0201-y. Bibcode: 2006JOM....58j..47W.
- ↑ Cooper, Alan (1999). "Joseph Black". History of Glasgow University Chemistry Department. University of Glasgow Department of Chemistry. http://www.chem.gla.ac.uk/dept/black.htm.
- ↑ Seyferth, Dietmar (2001). "Cadet's Fuming Arsenical Liquid and the Cacodyl Compounds of Bunsen". Organometallics 20 (8): 1488–1498. doi:10.1021/om0101947.
- ↑ Partington, J.R. (1989). A Short History of Chemistry. Dover Publications, Inc. ISBN 978-0-486-65977-0. https://archive.org/details/shorthistoryofch0000part_q6h4.
- ↑ Cavendish, Henry (1766). "Three Papers Containing Experiments on Factitious Air, by the Hon. Henry Cavendish". Philosophical Transactions (The University Press) 56: 141–184. doi:10.1098/rstl.1766.0019. Bibcode: 1766RSPT...56..141C. https://books.google.com/books?id=ygqYnSR3oe0C&q=the+scientific+papers+cavendish. Retrieved 6 November 2007.
- ↑ "Joseph Priestley". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Carl Wilhelm Scheele". History of Gas Chemistry. Center for Microscale Gas Chemistry, Creighton University. 2005-09-11. http://mattson.creighton.edu/History_Gas_Chemistry/Scheele.html.
- ↑ "Lavoisier, Antoine." Encyclopædia Britannica. 2007. Encyclopædia Britannica Online. 24 July 2007 <http://www.britannica.com/eb/article-9369846>.
- ↑ 41.0 41.1 41.2 Weisstein, Eric W. (1996). "Lavoisier, Antoine (1743–1794)". Eric Weisstein's World of Scientific Biography. Wolfram Research Products. http://scienceworld.wolfram.com/biography/Lavoisier.html.
- ↑ "Jacques Alexandre César Charles". Centennial of Flight. U.S. Centennial of Flight Commission. 2001. http://www.centennialofflight.gov/essay/Dictionary/Charles/DI16.htm.
- ↑ Burns, Ralph A. (1999). Fundamentals of Chemistry. Prentice Hall. p. 32. ISBN 978-0-02-317351-6. https://archive.org/details/fundamentalsofch0003burn.
- ↑ "Proust, Joseph Louis (1754–1826)". 100 Distinguished Chemists. European Association for Chemical and Molecular Science. 2005. http://www.euchems.org/Distinguished/19thCentury/proustlouis.asp.
- ↑ "Inventor Alessandro Volta Biography". The Great Idea Finder. 2005. http://www.ideafinder.com/history/inventors/volta.htm.
- ↑ 46.0 46.1 "John Dalton". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Joseph Louis Gay-Lussac". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "December 6 Births". Today in Science History. 2007. http://www.todayinsci.com/12/12_06.htm.
- ↑ "Jöns Jakob Berzelius". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Michael Faraday". Famous Physicists and Astronomers. http://www.phy.hr/~dpaar/fizicari/xfaraday.html.
- ↑ 51.0 51.1 51.2 "Justus von Liebig and Friedrich Wöhler". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "William Prout". http://www.cartage.org.lb/en/themes/Biographies/MainBiographies/P/Prout/1.html.
- ↑ "Hess, Germain Henri". http://www.cartage.org.lb/en/themes/Biographies/MainBiographies/H/Hess/1.html.
- ↑ "Kolbe, Adolph Wilhelm Hermann". 100 Distinguished European Chemists. European Association for Chemical and Molecular Sciences. 2005. http://www.euchems.org/Distinguished/19thCentury/kolbeadolph.asp.
- ↑ Weisstein, Eric W. (1996). "Kelvin, Lord William Thomson (1824–1907)". Eric Weisstein's World of Scientific Biography. Wolfram Research Products. http://scienceworld.wolfram.com/biography/Kelvin.html.
- ↑ "History of Chirality". Stheno Corporation. 2006. http://www.sthenocorp.com/history.htm.
- ↑ "Lambert-Beer Law". Sigrist-Photometer AG. 2007-03-07. http://www.photometer.com/en/abc/abc_061.htm.
- ↑ "Benjamin Silliman, Jr. (1816–1885)". Picture History. Picture History LLC. 2003. http://www.picturehistory.com/find/p/17879/mcms.html.
- ↑ "William Henry Perkin". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ 60.0 60.1 "Archibald Scott Couper and August Kekulé von Stradonitz". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ O'Connor, J. J.; Robertson, E.F. (2002). "Gustav Robert Kirchhoff". MacTutor. School of Mathematics and Statistics University of St Andrews, Scotland. http://www-groups.dcs.st-and.ac.uk/~history/Biographies/Kirchhoff.html.
- ↑ Eric R. Scerri, The Periodic Table: Its Story and Its Significance, Oxford University Press, 2006.
- ↑ "Alexander Parkes (1813–1890)". People & Polymers. Plastics Historical Society. http://www.plastiquarian.com/parkes.htm.
- ↑ 64.0 64.1 64.2 "The Periodic Table". The Third Millennium Online. http://www.3rd1000.com/history/periodic.htm.
- ↑ 65.0 65.1 "Julius Lothar Meyer and Dmitri Ivanovich Mendeleev". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ C.M. Guldberg and P. Waage,"Studies Concerning Affinity" C. M. Forhandlinger: Videnskabs-Selskabet i Christiana (1864), 35
- ↑ P. Waage, "Experiments for Determining the Affinity Law" ,Forhandlinger i Videnskabs-Selskabet i Christiania, (1864) 92.
- ↑ C.M. Guldberg, "Concerning the Laws of Chemical Affinity", C. M. Forhandlinger i Videnskabs-Selskabet i Christiania (1864) 111
- ↑ "No. 1858: Johann Josef Loschmidt". http://www.uh.edu/engines/epi1858.htm.
- ↑ "Adolf von Baeyer: The Nobel Prize in Chemistry 1905". Nobel Lectures, Chemistry 1901–1921. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1905/baeyer-bio.html.
- ↑ "Jacobus Henricus van't Hoff". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ O'Connor, J. J.; Robertson, E.F. (1997). "Josiah Willard Gibbs". MacTutor. School of Mathematics and Statistics University of St Andrews, Scotland. http://www-groups.dcs.st-and.ac.uk/~history/Biographies/Gibbs.html.
- ↑ Weisstein, Eric W. (1996). "Boltzmann, Ludwig (1844–1906)". Eric Weisstein's World of Scientific Biography. Wolfram Research Products. http://scienceworld.wolfram.com/biography/Boltzmann.html.
- ↑ "Svante August Arrhenius". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Jacobus H. van 't Hoff: The Nobel Prize in Chemistry 1901". Nobel Lectures, Chemistry 1901–1921. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1901/hoff-bio.html.
- ↑ "Emil Fischer: The Nobel Prize in Chemistry 1902". Nobel Lectures, Chemistry 1901–1921. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1902/fischer-bio.html.
- ↑ "Henry Louis Le Châtelier". World of Scientific Discovery. Thomson Gale. 2005. http://www.bookrags.com/biography/henry-louis-le-chatelier-wsd/. Retrieved 2007-03-24.
- ↑ "History of Chemistry". Intensive General Chemistry. Columbia University Department of Chemistry Undergraduate Program. http://www.columbia.edu/itc/chemistry/chem-c2507/navbar/chemhist.html.
- ↑ "Alfred Werner: The Nobel Prize in Chemistry 1913". Nobel Lectures, Chemistry 1901–1921. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1913/werner-bio.html.
- ↑ "William Ramsay: The Nobel Prize in Chemistry 1904". Nobel Lectures, Chemistry 1901–1921. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1904/ramsay-bio.html.
- ↑ "Joseph John Thomson". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Alfred Werner: The Nobel Prize in Physics 1911". Nobel Lectures, Physics 1901–1921. Elsevier Publishing Company. 1967. http://nobelprize.org/nobel_prizes/physics/laureates/1911/wien-bio.html.
- ↑ "Marie Sklodowska Curie". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ 84.0 84.1 "Ernest Rutherford: The Nobel Prize in Chemistry 1908". Nobel Lectures, Chemistry 1901–1921. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1908/rutherford-bio.html.
- ↑ "Tsvet, Mikhail (Semyonovich)". Compton's Desk Reference. Encyclopædia Britannica. 2007. http://deskreference.britannica.com/ebc/print?tocId=9381267. Retrieved 2007-03-24.
- ↑ "Physics Time-Line 1900 to 1949". Weburbia.com. http://www.weburbia.com/pg/hist3.htm.
- ↑ "Fritz Haber". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ Cassidy, David (1996). "Einstein on Brownian Motion". The Center for History of Physics. http://www.aip.org/history/einstein/essay-brownian.htm.
- ↑ "Leo Hendrik Baekeland". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Robert A. Millikan: The Nobel Prize in Physics 1923". Nobel Lectures, Physics 1922–1941. Elsevier Publishing Company. 1965. http://nobelprize.org/nobel_prizes/physics/laureates/1923/press.html.
- ↑ "Søren Sørensen". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ Parker, David. "Nuclear Twins: The Discovery of the Proton and Neutron". Electron Centennial Page. http://www.davidparker.com/janine/twins.html.
- ↑ "Solvay Conference". Einstein Symposium. 2005. http://www.bibalex.org/Einstein2005/About_Solvay.htm.
- ↑ "The Nobel Prize in Physics 1915". Nobelprize.org. The Nobel Foundation. http://nobelprize.org/nobel_prizes/physics/laureates/1915/.
- ↑ "Peter Debye: The Nobel Prize in Chemistry 1936". Nobel Lectures, Chemistry 1922–1941. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1936/debye-bio.html.
- ↑ "Niels Bohr: The Nobel Prize in Physics 1922". Nobel Lectures, Chemistry 1922–1941. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/physics/laureates/1922/bohr-bio.html.
- ↑ Weisstein, Eric W. (1996). "Moseley, Henry (1887–1915)". Eric Weisstein's World of Scientific Biography. Wolfram Research Products. http://scienceworld.wolfram.com/biography/Moseley.html.
- ↑ "Frederick Soddy The Nobel Prize in Chemistry 1921". Nobel Lectures, Chemistry 1901–1921. Elsevier Publishing Company. 1966. http://nobelprize.org/nobel_prizes/chemistry/laureates/1921/soddy-bio.html.
- ↑ "Early Mass Spectrometry". A History of Mass Spectrometry. Scripps Center for Mass Spectrometry. 2005. http://masspec.scripps.edu/MSHistory/timelines/1897.php.
- ↑ 100.0 100.1 "Gilbert Newton Lewis and Irving Langmuir". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Electron Spin". http://hyperphysics.phy-astr.gsu.edu/hbase/spin.html.
- ↑ LeMaster, Nancy; McGann, Diane (1992). "GILBERT NEWTON LEWIS: AMERICAN CHEMIST (1875–1946)". Woodrow Wilson Leadership Program in Chemistry. The Woodrow Wilson National Fellowship Foundation. http://www.woodrow.org/teachers/ci/1992/Lewis.html.
- ↑ "Louis de Broglie: The Nobel Prize in Physics 1929". Nobel Lectures, Physics 1922–1941. Elsevier Publishing Company. 1965. http://nobelprize.org/nobel_prizes/physics/laureates/1929/broglie-bio.html.
- ↑ "Wolfgang Pauli: The Nobel Prize in Physics 1945". Nobel Lectures, Physics 1942–1962. Elsevier Publishing Company. 1964. http://nobelprize.org/nobel_prizes/physics/laureates/1945/pauli-bio.html.
- ↑ "Erwin Schrödinger: The Nobel Prize in Physics 1933". Nobel Lectures, Physics 1922–1941. Elsevier Publishing Company. 1965. http://nobelprize.org/nobel_prizes/physics/laureates/1933/schrodinger-bio.html.
- ↑ "Werner Heisenberg: The Nobel Prize in Physics 1932". Nobel Lectures, Physics 1922–1941. Elsevier Publishing Company. 1965. http://nobelprize.org/nobel_prizes/physics/laureates/1932/heisenberg-bio.html.
- ↑ Heitler, Walter; London, Fritz (1927). "Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik". Zeitschrift für Physik 44 (6–7): 455–472. doi:10.1007/BF01397394. Bibcode: 1927ZPhy...44..455H.
- ↑ Ivor Grattan-Guinness. Companion Encyclopedia of the History and Philosophy of the Mathematical Sciences. Johns Hopkins University Press, 2003, p. 1266.; Jagdish Mehra, Helmut Rechenberg. The Historical Development of Quantum Theory. Springer, 2001, p. 540.
- ↑ 109.0 109.1 109.2 "Linus Pauling: The Nobel Prize in Chemistry 1954". Nobel Lectures, Chemistry 1942–1962. Elsevier. 1964. http://nobelprize.org/nobel_prizes/chemistry/laureates/1954/pauling-bio.html.
- ↑ Rzepa, Henry S.. "The aromaticity of Pericyclic reaction transition states". Department of Chemistry, Imperial College London. http://www.ch.ic.ac.uk/rzepa/pericyclic/.
- ↑ "Harold C. Urey: The Nobel Prize in Chemistry 1934". Nobel Lectures, Chemistry 1922–1941. Elsevier Publishing Company. 1965. http://nobelprize.org/nobel_prizes/chemistry/laureates/1934/urey-bio.html.
- ↑ "James Chadwick: The Nobel Prize in Physics 1935". Nobel Lectures, Physics 1922–1941. Elsevier Publishing Company. 1965. http://nobelprize.org/nobel_prizes/physics/laureates/1935/chadwick-bio.html.
- ↑ Jensen, William B. (2003). "Electronegativity from Avogadro to Pauling: II. Late Nineteenth- and Early Twentieth-Century Developments". Journal of Chemical Education 80 (3): 279. doi:10.1021/ed080p279. Bibcode: 2003JChEd..80..279J.
- ↑ "Wallace Hume Carothers". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Emilio Segrè: The Nobel Prize in Physics 1959". Nobel Lectures, Physics 1942–1962. Elsevier Publishing Company. 1965. http://nobelprize.org/nobel_prizes/physics/laureates/1959/segre-bio.html.
- ↑ "Eugene Houdry". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ "Pyotr Kapitsa: The Nobel Prize in Physics 1978". Les Prix Nobel, The Nobel Prizes 1991. Nobel Foundation. 1979. http://nobelprize.org/nobel_prizes/physics/laureates/1978/kapitsa-bio.html.
- ↑ "Otto Hahn: The Nobel Prize in Chemistry 1944". Nobel Lectures, Chemistry 1942–1962. Elsevier Publishing Company. 1964. http://nobelprize.org/nobel_prizes/chemistry/laureates/1944/hahn-bio.html.
- ↑ 119.0 119.1 "Glenn Theodore Seaborg". Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005.
- ↑ Seeman, Jeffrey I. (2007). "The Woodward-Doering/Rabe-Kindler total synthesis of quinine: setting the record straight". Angewandte Chemie International Edition 46 (9): 1378–1413. doi:10.1002/anie.200601551. ISSN 1433-7851. PMID 17294412. Bibcode: 2007ACIE...46.1378S.
- ↑ "The Nobel Prize in Physics 1952". Nobelprize.org. The Nobel Foundation. http://nobelprize.org/nobel_prizes/physics/laureates/1952/.
- ↑ "History of the Elements of the Periodic Table". AUS-e-TUTE. http://www.ausetute.com.au/elemhist.html.
- ↑ Hannaford, Peter. "Alan Walsh 1916–1998". AAS Biographical Memoirs. Australian Academy of Science. http://www.science.org.au/academy/memoirs/walsh2.htm.
- ↑ 124.0 124.1 Cornforth, Lord Todd, John; Cornforth, J.; T., A. R.; C., J. W. (November 1981). "Robert Burns Woodward. 10 April 1917-8 July 1979". Biographical Memoirs of Fellows of the Royal Society 27 (6): 628–695. doi:10.1098/rsbm.1981.0025. note: authorization required for web access.
- ↑ "The Nobel Prize in Medicine 1962". Nobelprize.org. The Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1962/.
- ↑ Skou, Jens (1957). "The influence of some cations on an adenosine triphosphatase from peripheral nerves.". Biochim Biophys Acta 23 (2): 394–401. doi:10.1016/0006-3002(57)90343-8. PMID 13412736.
- ↑ "The Nobel Prize in Chemistry 1962". Nobelprize.org. The Nobel Foundation. http://nobelprize.org/nobel_prizes/chemistry/laureates/1962/.
- ↑ "Neil Bartlett and the Reactive Noble Gases". American Chemical Society. http://portal.acs.org/portal/PublicWebSite/education/whatischemistry/landmarks/bartlettnoblegases/index.htm.
- ↑ G. A. Olah, S. J. Kuhn, W. S. Tolgyesi, E. B. Baker, J. Am. Chem. Soc. 1962, 84, 2733; G. A. Olah, lieu. Chim. (Bucharest), 1962, 7, 1139 (Nenitzescu issue); G. A. Olah, W. S. Tolgyesi, S. J. Kuhn, M. E. Moffatt, I. J. Bastien, E. B. Baker, J. Am. Chem. Soc. 1963, 85, 1328.
- ↑ "Richard R. Ernst The Nobel Prize in Chemistry 1991". Les Prix Nobel, The Nobel Prizes 1991. Nobel Foundation. 1992. http://nobelprize.org/nobel_prizes/chemistry/laureates/1991/ernst-autobio.html.
- ↑ H. Nozaki, S. Moriuti, H. Takaya, R. Noyori, Tetrahedron Lett. 1966, 5239;
- ↑ H. Nozaki, H. Takaya, S. Moriuti, R. Noyori, Tetrahedron 1968, 24, 3655.
- ↑ W. J. Hehre, W. A. Lathan, R. Ditchfield, M. D. Newton, and J. A. Pople, Gaussian 70 (Quantum Chemistry Program Exchange, Program No. 237, 1970).
- ↑ Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d'oléfines acycliques Die Makromolekulare Chemie Volume 141, Issue 1, Date: 9 February 1971, Pages: 161–176 Par Jean-Louis Hérisson, Yves Chauvin doi:10.1002/macp.1971.021410112
- ↑ Katsuki, Tsutomu (1980). "The first practical method for asymmetric epoxidation". Journal of the American Chemical Society 102 (18): 5974–5976. doi:10.1021/ja00538a077. Bibcode: 1980JAChS.102.5974K.
- ↑ Hill, J. G.; Sharpless, K. B.; Exon, C. M.; Regenye, R. Org. Synth., Coll. Vol. 7, p.461 (1990); Vol. 63, p.66 (1985). (Article)
- ↑ Jacobsen, Eric N. (1988). "Asymmetric dihydroxylation via ligand-accelerated catalysis". Journal of the American Chemical Society 110 (6): 1968–1970. doi:10.1021/ja00214a053. Bibcode: 1988JAChS.110.1968J.
- ↑ Kolb, Hartmuth C. (1994). "Catalytic Asymmetric Dihydroxylation". Chemical Reviews 94 (8): 2483–2547. doi:10.1021/cr00032a009.
- ↑ Gonzalez, J.; Aurigemma, C.; Truesdale, L. Org. Synth., Coll. Vol. 10, p.603 (2004); Vol. 79, p.93 (2002). (Article)
- ↑ Sharpless, K. Barry (1975). "New reaction. Stereospecific vicinal oxyamination of olefins by alkyl imido osmium compounds". Journal of the American Chemical Society 97 (8): 2305–2307. doi:10.1021/ja00841a071. Bibcode: 1975JAChS..97.2305S.
- ↑ Herranz, Eugenio (1978). "Osmium-catalyzed vicinal oxyamination of olefins by N-chloro-N-argentocarbamates". Journal of the American Chemical Society 100 (11): 3596–3598. doi:10.1021/ja00479a051. Bibcode: 1978JAChS.100.3596H.
- ↑ Herranz, E.; Sharpless, K. B. Org. Synth., Coll. Vol. 7, p.375 (1990); Vol. 61, p.85 (1983). (Article)
- ↑ "The Nobel Prize in Chemistry 1996". Nobelprize.org. The Nobel Foundation. http://nobelprize.org/nobel_prizes/chemistry/laureates/1996/.
- ↑ "Benjamin Franklin Medal awarded to Dr. Sumio Iijima, Director of the Research Center for Advanced Carbon Materials, AIST". National Institute of Advanced Industrial Science and Technology. 2002. http://www.aist.go.jp/aist_e/topics/20020129/20020129.html.
- ↑ First total synthesis of taxol 1. Functionalization of the B ring Robert A. Holton, Carmen Somoza, Hyeong Baik Kim, Feng Liang, Ronald J. Biediger, P. Douglas Boatman, Mitsuru Shindo, Chase C. Smith, Soekchan Kim, et al.; J. Am. Chem. Soc.; 1994; 116(4); 1597–1598. DOI Abstract
- ↑ Holton, Robert A. (1994). "First total synthesis of taxol. 2. Completion of the C and D rings". Journal of the American Chemical Society 116 (4): 1599–1600. doi:10.1021/ja00083a067. Bibcode: 1994JAChS.116.1599H.
- ↑ Holton, Robert A. (1988). "A synthesis of taxusin". Journal of the American Chemical Society 110 (19): 6558–6560. doi:10.1021/ja00227a043. Bibcode: 1988JAChS.110.6558H.
- ↑ "Cornell and Wieman Share 2001 Nobel Prize in Physics". NIST News Release. National Institute of Standards and Technology. 2001. https://www.nist.gov/public_affairs/releases/n01-04.htm.
Further reading
- Crossing Oceans: Exchange of Products, Instruments, Procedures and Ideas in the History of Chemistry and Related Sciences, edited by Ana Maria Alfonso-Goldfarb, Walter Carnielli, Hasok Chang, Márcia H. M. Ferraz, & Silvia Waisse. São Paulo, Brazil: Coleção CLE/UNICAMP, 2015.
- Servos, John W., Physical chemistry from Ostwald to Pauling : the making of a science in America, Princeton, N.J. : Princeton University Press, 1990. ISBN 0-691-08566-8
External links
- Eric Weisstein's World of Scientific Biography
- History of Gas Chemistry
- list of all Nobel Prize laureates
- History of Elements of the Periodic Table
- CHEM-HIST: International Mailing List for the History of Chemistry
Template:Featured list is only for Wikipedia:Featured lists.
 |
