Chemistry:Ytterbium(II) iodide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| I2Yb | |
| Molar mass | 426.854 g·mol−1 |
| Appearance | yellow solid[1] |
| Melting point | 780 °C (1,440 °F; 1,050 K)[1] (decomposes) |
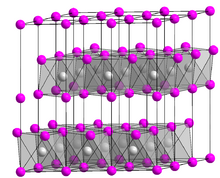
| Structure | |
| Trigonal | |
| P3m1 (No. 164) | |
a = 448 pm, c = 696 pm
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ytterbium(II) iodide is an iodide of ytterbium, with the chemical formula of YbI2. It is a yellow solid.
Preparation
Ytterbium(II) iodide can be prepared by heating ytterbium(III) iodide:[1]
- [math]\displaystyle{ \mathrm{2\ YbI_3\ \xrightarrow []{\Delta\ T}\ 2\ YbI_2\ +\ I_2} }[/math]
It can also be prepared by reacting metallic ytterbium with 1,2-diiodoethane in tetrahydrofuran:[2]
- [math]\displaystyle{ \mathrm{Yb\ +\ ICH_2CH_2I\ \xrightarrow []{THF} \ YbI_2\ +\ H_2C=CH_2} }[/math]
Although the reaction takes place at room temperature, due to the sensitivity of the reagents it is necessary to work anhydrous and under inert gas. Otherwise, if oxygen is present, rapid oxidation to ytterbium(III) takes place. This can be visually recognized by the color change from green to yellow solution.
Properties and uses
Ytterbium(II) iodide is a yellow solid that is very sensitive to air and moisture and is rapidly oxidized to ytterbium(III). It reacts with water to produce hydrogen gas and basic iodides, and reacts violently with acids.[1] Ytterbium(II) iodide sinters at 0.01 Torr from about 780 °C and gives a viscous melt at about 920 °C. It begins to disproportionate into ytterbium and ytterbium(III) iodide. At around 800 °C, a yellow sublimate of ytterbium(II) iodide is observed on the glass walls; this partly obscures the disproportionation. The melting point can therefore only be determined imprecisely.[1][3]
Like samarium(II) iodide (SmI2), ytterbium(II) iodide is a reagent used in organic chemical reactions.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 G. Jantsch; N. Skalla; H. Jawurek (1931-11-10). "Zur Kenntnis der Halogenide der seltenen Erden. V. Über die Halogenide des Ytterbiums" (in en). Zeitschrift für anorganische und allgemeine Chemie 201 (1): 207–220. doi:10.1002/zaac.19312010119.
- ↑ 2.0 2.1 Pierre-Marie Girard; Jean Louis Namy; Henri B. Kagan (April 1980). "Divalent lanthanide derivatives in organic synthesis. 1. Mild preparation of samarium iodide and ytterbium iodide and their use as reducing or coupling agents" (in en). Journal of the American Chemical Society 102 (8): 2693–2698. doi:10.1021/ja00528a029. ISSN 0002-7863.
- ↑ Gmelins Handbuch der anorganischen Chemie, System Nr. 39, Band C 6, S. 199–200.
Further reading
- Kagan, Henri B.; Namy, Jean Louis (1986). "Tetrahedron report number 213: Lanthanides in organic synthesis". Tetrahedron 42 (24): 6573–6614. doi:10.1016/s0040-4020(01)82098-6. ISSN 0040-4020.
- Steel, Patrick G. (2001-10-18). "Recent developments in lanthanide mediated organic synthesis". Journal of the Chemical Society, Perkin Transactions 1 (21): 2727–2751. doi:10.1039/a908189e. ISSN 1472-7781.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

