Chemistry:Nickel(II) bromide
| |
 Anhydrous
| |
 Hexahydrate
| |
| Names | |
|---|---|
| IUPAC name
Nickel(II) bromide
| |
| Other names
Nickel dibromide,
Nickel bromide, Nickelous bromide | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| NiBr2 | |
| Molar mass | 218.53 g/mol |
| Appearance | yellow-brown crystals |
| Odor | odorless |
| Density | 5.10 g/cm3[1] |
| Melting point | 963 °C (1,765 °F; 1,236 K) sublimes[1] |
| 1.13 kg/L (0 °C) 1.22 kg/L (10 °C) 1.31 kg/L (20 °C)[1] 1.44 kg/L (40 °C) 1.55 kg/L (100 °C)[2] | |
| Band gap | 2.5 eV[3] |
| +5600.0·10−6 cm3/mol[4] | |
| Structure[5] | |
| hexagonal, hR9 | |
| R3m, No. 166 | |
a = 0.36998 nm, c = 1.82796 nm
| |
Formula units (Z)
|
3 |
| Thermochemistry[6] | |
Std enthalpy of
formation (ΔfH⦵298) |
−212.1 kJ·mol−1 |
| Hazards | |
| Main hazards | Irritant, corrosive |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
nickel(II) fluoride nickel(II) chloride nickel(II) iodide |
Other cations
|
cobalt(II) bromide copper(II) bromide palladium(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
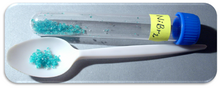
Nickel(II) bromide is the name for the inorganic compounds with the chemical formula NiBr2(H2O)x. The value of x can be 0 for the anhydrous material, as well as 2, 3, or 6 for the three known hydrate forms. The anhydrous material is a yellow-brown solid which dissolves in water to give blue-green hexahydrate (see picture).
Structure
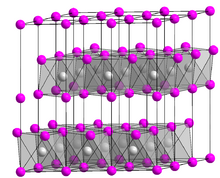
The structure of the nickel bromides varies with the degree of hydration. In all of these cases, the nickel(II) ion adopts an octahedral molecular geometry. Similar structures are observed in aqueous solutions of nickel bromide.[7]
- Anhydrous NiBr2 adopts the hexagonal cadmium chloride structure.[8] The interatomic distance for Ni-Br is 2.52—2.58 Å.[7] Anhydrous NiBr2 is a paramagnet at room temperature. Upon cooling, it turns into an antiferromagnet at 52 K, and then into a helimagnet at 22.8 K.[5]
- The structure of the trihydrate has not been confirmed by X-ray crystallography. It is assumed to adopt a chain structure.[9]
- The di- and hexahydrates adopt structures akin to those for the corresponding chlorides. The dihydrate consists of a linear chain, whereas the hexahydrate features isolated trans-[NiBr2(H2O)4] molecules together with two water molecules of crystallization.
Reactions and uses
NiBr2 has Lewis acid character, as indicated by its tendency to hydrate and form adducts with a variety of other Lewis bases.
NiBr2 is also used to prepare catalysts for cross-coupling reactions and various carbonylations.[8] NiBr2-glyme shows increased activity compared to NiCl2-glyme for some transformations.[10] File:NiBr2 scheme.tif
Safety
This compound is a suspected carcinogen.[11]
References
- ↑ 1.0 1.1 1.2 Haynes, p. 4.73
- ↑ nickel(II) bromide. chemister.ru
- ↑ Lee, Geunseop; Oh, S.-J. (1991). "Electronic structures of NiO, CoO, and FeO studied by 2pcore-level x-ray photoelectron spectroscopy". Physical Review B 43 (18): 14674–14682. doi:10.1103/PhysRevB.43.14674. PMID 9997359. Bibcode: 1991PhRvB..4314674L.
- ↑ Haynes, p. 4.129
- ↑ 5.0 5.1 Nasser, J.A.; Kiat, J.M.; Gabilly, R. (1992). "X-ray investigation of magnetostriction in NiBr2". Solid State Communications 82 (1): 49–54. doi:10.1016/0038-1098(92)90404-W. Bibcode: 1992SSCom..82...49N.
- ↑ Haynes, p. 5.29
- ↑ 7.0 7.1 Wakita, Hisanobu; Ichihashi, Mitsuyoshi; Mibuchi, Takeharu; Masuda, Isao (1982). "The Structure of Nickel(II) Bromide in Highly Concentrated Aqueous Solution by X-Ray Diffraction Analysis". Bulletin of the Chemical Society of Japan 55 (3): 817–821. doi:10.1246/bcsj.55.817.
- ↑ 8.0 8.1 Luh, Tien-Yau; Kuo, Chi-Hong (2001-01-01). Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289X.rn009. ISBN 9780470842898.
- ↑ Defotis, G. C.; Goodey, J. R.; Narducci, A. A.; Welch, M. H. (1996). "NiBr2·3H2O, a lower dimensional antiferromagnet". Journal of Applied Physics 79 (8): 4718. doi:10.1063/1.361651.
- ↑ Konev, Mikhail O.; Hanna, Luke E.; Jarvo, Elizabeth R. (2016-06-01). "Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides" (in en). Angewandte Chemie International Edition 55 (23): 6730–6733. doi:10.1002/anie.201601206. PMID 27099968.
- ↑ "NICKEL BROMIDE | CAMEO Chemicals | NOAA". https://cameochemicals.noaa.gov/chemical/8879.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
 |


