Chemistry:Dichlorine hexoxide
| |||
|
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dichlorine hexoxide
| |||
| Other names
Chlorine trioxide; Chloryl perchlorate; Chlorine(V,VII) oxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider |
| ||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| Cl2O6 | |||
| Molar mass | 166.901 g/mol | ||
| Appearance | red liquid | ||
| Density | 1.65 g/cm3 | ||
| Melting point | 3.5 °C (38.3 °F; 276.6 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) | ||
| Reacts | |||
| Hazards | |||
| Main hazards | oxidizer | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
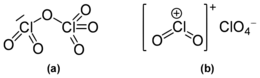
Dichlorine hexoxide is the chemical compound with the molecular formula Cl2O6, which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate [ClO2]+[ClO4]−, which may be thought of as the mixed anhydride of chloric and perchloric acids.
It is produced by reaction between chlorine dioxide and excess ozone:
- 2 ClO2 + 2 O3 → 2 ClO3 + 2 O2 → Cl2O6 + 2 O2
Molecular structure
It was originally reported to exist as the monomeric chlorine trioxide ClO3 in gas phase,[1] but was later shown to remain an oxygen-bridged dimer after evaporation and until thermal decomposition into chlorine perchlorate, Cl2O4, and oxygen.[2] The compound ClO3 was then rediscovered.[3]
It is a dark red fuming liquid at room temperature that crystallizes as a red ionic compound, chloryl perchlorate, [ClO2]+[ClO4]−. The red color shows the presence of chloryl ions. Thus, chlorine's formal oxidation state in this compound remains a mixture of chlorine (V) and chlorine (VII) both in the gas phase and when condensed; however by breaking one oxygen-chlorine bond some electron density does shifts towards the chlorine (VII).
Properties
Cl2O6 is diamagnetic and is a very strong oxidizing agent. Although stable at room temperature, it explodes violently on contact with organic compounds[4] and reacts with gold to produce the chloryl salt [ClO2]+[Au(ClO4)4]−.[5] Many other reactions involving Cl2O6 reflect its ionic structure, [ClO2]+[ClO4]−, including the following:[6]
- NO2F + Cl2O6 → NO2ClO4 + ClO2F
- NO + Cl2O6 → NOClO4 + ClO2
- 2 V2O5 + 12 Cl2O6 → 4 VO(ClO4)3 + 12 ClO2 + 3 O2
- SnCl4 + 6 Cl2O6 → [ClO2]2[Sn(ClO4)6] + 4 ClO2 + 2 Cl2
- 2Au + 6Cl2O6 → 2[ClO2]+[Au(ClO4)4]− + Cl2
Nevertheless, it can also react as a source of the ClO3 radical:
- 2 AsF5 + Cl2O6 → 2 ClO3AsF5
References
- ↑ C. F. Goodeve, F. A. Todd (1933). "Chlorine Hexoxide and Chlorine Trioxide". Nature 132 (3335): 514–515. doi:10.1038/132514b0. Bibcode: 1933Natur.132..514G.
- ↑ Lopez, Maria; Juan E. Sicre (1990). "Physicochemical properties of chlorine oxides. 1. Composition, ultraviolet spectrum, and kinetics of the thermolysis of gaseous dichlorine hexoxide". J. Phys. Chem. 94 (9): 3860–3863. doi:10.1021/j100372a094.
- ↑ Grothe, Hinrich; Willner, Helge (1994). "Chlorine Trioxide: Spectroscopic Properties, Molecular Structure, and Photochemical Behavior". Angew. Chem. Int. Ed. 33 (14): 1482–1484. doi:10.1002/anie.199414821.
- ↑ Mary Eagleson (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 215. ISBN 3-11-011451-8. https://archive.org/details/conciseencyclope00eagl.
- ↑ Cunin, Frédérique; Catherine Deudon; Frédéric Favier; Bernard Mula; Jean Louis Pascal (2002). "First anhydrous gold perchlorato complex: ClO2Au(ClO4)4. Synthesis and molecular and crystal structure analysis". Inorganic Chemistry 41 (16): 4173–4178. doi:10.1021/ic020161z. PMID 12160405.
- ↑ Harry Julius Emeléus, Alan George Sharpe (1963). Advances in Inorganic Chemistry and Radiochemistry. Academic Press. p. 65. ISBN 0-12-023605-2. https://books.google.com/books?id=pRXIwIV-hB8C.
 |