Chemistry:Rubidium triiodide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| RbI3 | |
| Appearance | black crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rubidium triiodide is an inorganic compound with the chemical formula RbI3. It is composed of Rb+ and I−3.
Preparation
Rubidium triiodide can be obtained by heating rubidium iodide and iodine in aqueous solution:[1]
- RbI + I2 → RbI3
Properties
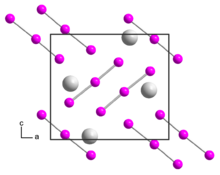
Rubidium triiodide is an orthorhombic black crystal, isomorphic to caesium triiodide, with space group Pnma, unit cell parameters a = 1090.8 pm, b = 665.5 pm, c = 971.1 pm.[2] Upon heating to 270 °C, the rubidium triiodide decomposes into rubidium iodide and elemental iodine.[1] It is soluble in ethanol and decomposes in ether.[1]
Reactions
It has long been believed that rubidium triiodide reacts further with iodine to form RbI7 and RbI9,[3] but this has been refuted by recent studies.[4][2]
References
- ↑ 1.0 1.1 1.2 Wells, H. L.; Wheeler, H. L.; Penfield, S. L. (1892). "Über Trihalogenverbindungen des Rubidiums und Kaliums. nebst ihrer Krystallographie" (in en). Zeitschrift für anorganische Chemie 1 (1): 442–455. doi:10.1002/zaac.18920010140. https://onlinelibrary.wiley.com/doi/10.1002/zaac.18920010140.
- ↑ 2.0 2.1 Tebbe, K. F.; Georgy, U. (1986-12-15). "Die Kristallstruckturen von Rubidiumtriiodid und Thalliumtriiodid". Acta Crystallographica Section C Crystal Structure Communications (International Union of Crystallography (IUCr)) 42 (12): 1675–1678. doi:10.1107/s0108270186090972. ISSN 0108-2701.
- ↑ Abegg, R. (1906-09-21). "Über die festen Polyjodide der Alkalien, ihre Stabilität und Existenzbedingungen bei 25°". Zeitschrift für anorganische Chemie (Wiley) 50 (1): 403–438. doi:10.1002/zaac.19060500136. ISSN 0863-1778. https://zenodo.org/record/2048953.
- ↑ Briggs, T. R.; Patterson, E. S. (1932-10-01). "The Polyiodides of Rubidium. I". The Journal of Physical Chemistry (American Chemical Society (ACS)) 36 (10): 2621–2624. doi:10.1021/j150340a011. ISSN 0092-7325.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

