Chemistry:Tellurium tetraiodide
| |
| Names | |
|---|---|
| Other names
tellurium(IV) iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| TeI4 | |
| Molar mass | 635.218 g/mol |
| Appearance | black crystals |
| Density | 5.05 g/cm3, solid |
| Melting point | 280 °C (536 °F; 553 K) |
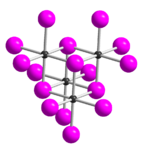
| Structure | |
| orthorhombic | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H312, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P322, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4.[2] In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.[2]
Preparation
Tellurium tetraiodide can be prepared by reacting Te and iodomethane, CH3I.[2] In the vapour TeI4 dissociates:[3]
- TeI4 → TeI2 + I2
It can be also obtained by reacting telluric acid with hydrogen iodide.[4]
- Te(OH)6 + HI → TeI4 + I2 + 6 H2O
It can also be obtained by reacting the elements, which can also produce tellurium diiodide and tellurium monoiodide, depending on the reaction conditions:[5]
- Te + 2 I2 → TeI4
- TeI4 → TeI2 + I2
Properties
Tellurium tetraiodide is an iron-gray solid that decomposes slowly in cold water and quickly in warm water to form tellurium dioxide and hydrogen iodide.[6] It is stable even in moist air and decomposes when heated, releasing iodine. It is soluble in hydriodic acid to form H[TeI5] and it is slightly soluble in acetone.[4]
Tellurium tetraiodide is a conductor when molten, dissociating into the ions TeI3+ and I−. In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:[3]
- TeI4 + 2 CH3CN → (CH3CN)2TeI3+ + I−
Five modifications of tellurium tetraiodide are known, all of which are composed of tetrameric molecules.[7] The δ form is the most thermodynamically stable form. This is structurally derived (as well as the α, β and γ forms) from the ε form.
References
- ↑ "Tellurium tetraiodide" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/82255#section=Safety-and-Hazards.
- ↑ 2.0 2.1 2.2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 3.0 3.1 Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN:0-12-352651-5
- ↑ 4.0 4.1 Handbuch der präparativen anorganischen Chemie. 1 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1975. p. 435. ISBN 978-3-432-02328-1.
- ↑ Hagen, A. P. (2009-09-17) (in en). Inorganic Reactions and Methods, The Formation of Bonds to Halogens (Part 1). John Wiley & Sons. ISBN 978-0-470-14538-8. https://books.google.com/books?id=zVIpX7rkk7oC&pg=PA59.
- ↑ Tellurium(IV) iodide, 99% (metals basis) at AlfaAesar, accessed on 2013-12-17 (PDF) (JavaScript required).
- ↑ Riedel, Erwin; Janiak, Christoph (2011). Anorganische Chemie: Zusatzmaterial online. Studium (8. Aufl ed.). Berlin: de Gruyter. ISBN 978-3-11-022567-9.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

