Chemistry:Vemurafenib
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌvɛməˈræfənɪb/ VEM-ə-RAF-ə-nib |
| Trade names | Zelboraf |
| Other names | PLX4032, RG7204, PLX4720, RO5185426 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
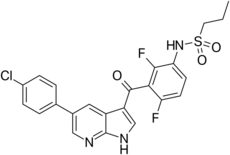
| Formula | C23H18ClF2N3O3S |
| Molar mass | 489.92 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
| vemurafenib | |
|---|---|
| Drug mechanism | |
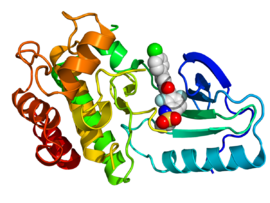 Crystallographic structure of B-Raf (rainbow colored, N-terminus = blue, C-terminus = red) complexed with vemurafenib (spheres, carbon = white, oxygen = red, nitrogen = blue, chlorine = green, fluorine = cyan, sulfur = yellow).[2] | |
| Therapeutic use | melanoma |
| Biological target | BRAF |
| Mechanism of action | protein kinase inhibitor |
| External links | |
| PDB ligand id | 032: PDBe, RCSB PDB |
| LIGPLOT | 3og7 |
Vemurafenib (INN), sold under the brand name Zelboraf, is a medication used for the treatment of late-stage melanoma.[2] It is an inhibitor of the B-Raf enzyme and was developed by Plexxikon.[2]
Mechanism of action
Vemurafenib causes programmed cell death in melanoma cell lines.[3] Vemurafenib interrupts the B-Raf/MEK step on the B-Raf/MEK/ERK pathway − if the B-Raf has the common V600E mutation.
Vemurafenib only works in melanoma patients whose cancer has a V600E BRAF mutation (that is, at amino acid position number 600 on the B-Raf protein, the normal valine is replaced by glutamic acid).[4] About 60% of melanomas have this mutation. It also has efficacy against the rarer BRAF V600K mutation. Melanoma cells without these mutations are not inhibited by vemurafenib; the drug paradoxically stimulates normal BRAF and may promote tumor growth in such cases.[5][6]
Resistance
Three mechanisms of resistance to vemurafenib (covering 40% of cases) have been discovered:
- Cancer cells begin to overexpress cell surface protein PDGFRB, creating an alternative survival pathway.
- A second oncogene called NRAS mutates, reactivating the normal BRAF survival pathway.[7]
- Stromal cell secretion of hepatocyte growth factor (HGF).[8][9]
Side effects
At the maximum tolerated dose (MTD) of 960 mg twice a day 31% of patients get skin lesions that may need surgical removal.[2] The BRIM-2 trial investigated 132 patients; the most common adverse events were arthralgia in 58% of patients, skin rash in 52%, and photosensitivity in 52%. In order to better manage side effects some form of dose modification was necessary in 45% of patients. The median daily dose was 1750 mg, 91% of the MTD.[10]
History
In a phase I clinical study, vemurafenib (then known as PLX4032) was able to reduce numbers of cancer cells in over half of a group of 16 patients with advanced melanoma. The treated group had a median increased survival time of 6 months over the control group.[11][12][13][14]
A second phase I study, in patients with a V600E mutation in B-Raf, ~80% showed partial to complete regression. The regression lasted from 2 to 18 months.[15]
In early 2010 a Phase I trial[16] for solid tumors (including colorectal cancer), and a phase II study (for metastatic melanoma) were ongoing.[17]
A phase III trial (vs dacarbazine) in patients with previously untreated metastatic melanoma showed an improved rates of overall and progression-free survival.[18]
In June 2011, positive results were reported from the phase III BRIM3 BRAF-mutation melanoma study.[19] The BRIM3 trial reported good updated results in 2012.[20]
Further trials are planned including a trial of vemurafenib co-administered with GDC-0973 (cobimetinib), a MEK-inhibitor.[19] After good results in 2014, the combination was submitted to the European Medical Agency and the US Food and Drug Administration for marketing approval.[21]
In January 2015, trial results compared vemurafenib with the combination of dabrafenib and trametinib for metastatic melanoma.[22]
Society and culture
Legal status
Vemurafenib was approved in the United States for the treatment of late-stage melanoma in August 2011,[23] making it the first drug designed using fragment-based lead discovery to gain regulatory approval.[24]
Vemurafenib was approved for use in Canada in February 2012.[25]
In February 2012, the European Commission approved vemurafenib as a monotherapy for the treatment of adults with BRAF V600E mutation positive unresectable or metastatic melanoma, the most aggressive form of skin cancer.[26]
In November 2017, the US Food and Drug Administration (FDA) approved vemurafenib for the treatment of people with Erdheim–Chester disease (ECD), a rare type of histiocytic neoplasm.[27][28]
Research
A trial combining vemurafenib and ipilimumab was stopped in April 2013 because of signs of liver toxicity.[29]
Treating Hairy Cell Leukemia
In 2012, a grant from the Hairy cell leukemia Foundation supported the discovery of the BRAF mutation in classic HCL. This discovery charted a new path forward for many patients. It improved diagnosis and opened the door for additional therapies to be used in managing HCL.[30] In a phase II clinical trial, Memorial Sloan Kettering is testing Vemurafenib, plus Obinutuzumab, in patients with previously untreated classical hairy cell leukemia.[31] A separate clinical study treatment with only Vemurafenib (or monotherarpy) demonstrated high response rates in relapsed/refractory (R/R) hairy cell leukemia (HCL), achieving an overall response rate of 86%, including 33% complete response (CR) and 53% partial response. However, after a median follow-up of 40 months, 21 of 31 responders (68%) experienced relapse with a median relapse-free survival (RFS) of 19 months (range, 12.5-53.9 months).[32]
References
- ↑ 1.0 1.1 "Australian Product Information: Zelboraf® (vemurafenib)". Roche Products Pty Limited. 25 March 2020. https://www.guildlink.com.au/gc/ws/ro/pi.cfm?product=ropzelbo10615.
- ↑ 2.0 2.1 2.2 2.3 PDB: 3OG7; "Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma". Nature 467 (7315): 596–599. September 2010. doi:10.1038/nature09454. PMID 20823850. Bibcode: 2010Natur.467..596B.
- ↑ "BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells". Molecular Cancer Research 6 (5): 751–759. May 2008. doi:10.1158/1541-7786.MCR-07-2001. PMID 18458053.
- ↑ "Metastatic melanoma - a review of current and future treatment options". Acta Dermato-Venereologica 95 (5): 516–524. May 2015. doi:10.2340/00015555-2035. PMID 25520039.
- ↑ "RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth". Nature 464 (7287): 431–435. March 2010. doi:10.1038/nature08833. PMID 20130576. Bibcode: 2010Natur.464..431H.
- ↑ "PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells". Pigment Cell & Melanoma Research 23 (2): 190–200. April 2010. doi:10.1111/j.1755-148X.2010.00685.x. PMID 20149136.
- ↑ "Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation". Nature 468 (7326): 973–977. December 2010. doi:10.1038/nature09626. PMID 21107323. Bibcode: 2010Natur.468..973N.
- "Researchers Uncover Drug-Resistance Mechanisms in BRAF Melanoma". Genetic Engineering & Biotechnology News. November 25, 2010. http://www.genengnews.com/gen-news-highlights/researchers-uncover-drug-resistance-mechanisms-in-braf-melanoma/81244289/.
- ↑ "Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion". Nature 487 (7408): 500–504. July 2012. doi:10.1038/nature11183. PMID 22763439. Bibcode: 2012Natur.487..500S.
- ↑ "Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors". Nature 487 (7408): 505–509. July 2012. doi:10.1038/nature11249. PMID 22763448. Bibcode: 2012Natur.487..505W.
- ↑ "BRIM-2 Upholds Benefits Emerging with Vemurafenib in Melanoma". Oncology & Biotech News 5 (7). July 2011. http://www.onclive.com/publications/obtn/2011/july-2011/BRIM-2-Upholds-Benefits-Emerging-with-Vemurafenib-in-Melanoma.
- ↑ "Drug hope for advanced melanoma". BBC News. 2009-06-02. http://news.bbc.co.uk/2/hi/health/8076743.stm.
- ↑ "A Roller Coaster Chase for a Cure". The New York Times. 2010-02-21. https://www.nytimes.com/2010/02/22/health/research/22trial.html.
- ↑ "Cancer research. Melanoma drug vindicates targeted approach". Science 326 (5960): 1619. December 2009. doi:10.1126/science.326.5960.1619. PMID 20019269. Bibcode: 2009Sci...326.1619G.
- ↑ "Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer". 2009 ASCO Annual Meeting Abstract, J Clin Oncol 27:15s, 2009 (suppl; abstr 9000). http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=34909.
- ↑ "Inhibition of mutated, activated BRAF in metastatic melanoma". The New England Journal of Medicine 363 (9): 809–819. August 2010. doi:10.1056/NEJMoa1002011. PMID 20818844.
- ↑ Clinical trial number NCT00405587 for "Safety Study of PLX4032 in Patients With Solid Tumors" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00949702 for "A Study of RO5185426 in Previously Treated Patients With Metastatic Melanoma" at ClinicalTrials.gov
- ↑ "Plexxikon Announces First Patient Dosed in Phase 3 Trial of PLX4032 (RG7204) for Metastatic Melanoma" (Press release). Plexxikon. 2010-01-08. Archived from the original on 2020-12-01. Retrieved 2011-02-03.
- ↑ 19.0 19.1 "Plexxikon and Roche Report Positive Data from Phase III BRAF Mutation Melanoma Study". Genetic Engineering & Biotechnology News (Mary Ann Liebert Publishers). 6 June 2011. http://www.genengnews.com/gen-news-highlights/plexxikon-and-roche-report-positive-data-from-phase-iii-braf-mutation-melanoma-study/81245246/.
- ↑ "Vemurafenib Improves Overall Survival in Patients with Metastatic Melanoma". http://www.chemotherapyadvisor.com/vemurafenib-improves-overall-survival-in-patients-with-metastatic-melanoma/article/244197/.
- ↑ "Cobimetinib at exelixis.com". http://www.exelixis.com/pipeline/GDC_0973_xl518.
- ↑ "MEK/BRAF Inhibitor Combo Reduces Death by One-Third in Melanoma". 2015. https://www.cancernetwork.com/view/mekbraf-inhibitor-combo-reduces-death-one-third-melanoma.
- ↑ "FDA Approves Zelboraf (Vemurafenib) and Companion Diagnostic for BRAF Mutation-Positive Metastatic Melanoma, a Deadly Form of Skin Cancer" (Press release). Genentech. Retrieved 2011-08-17.
- ↑ "Vemurafenib: the first drug approved for BRAF-mutant cancer". Nature Reviews. Drug Discovery 11 (11): 873–886. November 2012. doi:10.1038/nrd3847. PMID 23060265.
- ↑ "Notice of Decision for ZELBORAF". Health Canada. 11 March 2012. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/nd_ad_2012_zelboraf_148693-eng.php.
- ↑ "First Personalized Cancer Medicine Allows Patients with Deadly Form of Metastatic Melanoma to Live Significantly Longer". Onco'Zine. The International Cancer Network. February 20, 2012. http://oncozine.com/profiles/blogs/first-personalized-cancer-medicine-allows-patients-deadly-form-of.
- ↑ "FDA approves first treatment for certain patients with Erdheim–Chester disease, a rare blood cancer". U.S. Food and Drug Administration (FDA) (Press release). Retrieved 2018-05-20.
- ↑ "Vemurafenib for BRAF V600-Mutant Erdheim-Chester Disease and Langerhans Cell Histiocytosis: Analysis of Data From the Histology-Independent, Phase 2, Open-label VE-BASKET Study". JAMA Oncology 4 (3): 384–388. March 2018. doi:10.1001/jamaoncol.2017.5029. PMID 29188284.
- ↑ "Getting close and personal". The Economist. January 4, 2014. ISSN 0013-0613. https://www.economist.com/news/science-and-technology/21592599-researchers-and-drug-companies-are-ganging-up-new-push-against.
- ↑ "Hairy Cell Leukemia: Celebrating Progress?". July 29, 2022. https://www.hairycellleukemia.org/blog/campaignlaunch.
- ↑ Clinical trial number NCT03410875 for "Hairy Cell Leukemia with Vemurafebib" at ClinicalTrials.gov
- ↑ "Long-term outcomes in patients with relapsed or refractory hairy cell leukemia treated with vemurafenib monotherapy". Blood 140 (25): 2663–2671. December 2022. doi:10.1182/blood.2022016183. PMID 35930750.
Further reading
- "Vemurafenib Therapy and BRAF and NRAS Genotype". Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). 2017. Bookshelf ID: NBK447416. https://www.ncbi.nlm.nih.gov/books/NBK447416/.
External links
- "Vemurafenib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/vemurafenib.
 |

