Chemistry:Certolizumab pegol
From HandWiki
Short description: Pharmaceutical drug
 | |
 Syringe with 200mg Certolizumab pegol | |
| Monoclonal antibody | |
|---|---|
| Type | Fab' fragment |
| Source | Humanized (from mouse) |
| Target | TNF alpha |
| Clinical data | |
| Trade names | Cimzia |
| Other names | CDP870 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608041 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | about 11 days |
| Excretion | Kidney (PEG only) |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C2115H3252N556O673S16 |
| Molar mass | 47749.46 g·mol−1 |
| | |
Certolizumab pegol, sold under the brand name Cimzia, is a biopharmaceutical medication for the treatment of Crohn's disease,[1][2] rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.[3][4][5]
It is on the World Health Organization's List of Essential Medicines.[6]
Medical uses
- Crohn's Disease
- On April 22, 2008, the U.S. Food and Drug Administration (FDA) approved Cimzia for the treatment of Crohn's disease in people who did not respond sufficiently or adequately to standard therapy.[4][7][8]
- Rheumatoid arthritis
- On June 26, 2009, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a positive opinion recommending that the European Commission grant a marketing authorisation for Cimzia for the treatment of rheumatoid arthritis only - the CHMP refused approval for the treatment of Crohn's disease. The marketing authorisation was granted to UCB Pharma SA on October 1, 2009.[9]
- Psoriatic arthritis
- On September 27, 2013, the U.S. FDA approved Cimzia for the treatment of adult patients with active psoriatic arthritis.[10]
Method of action

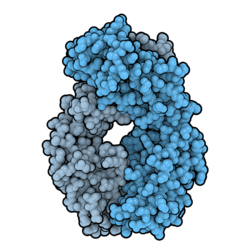
Three certolizumab molecules (blue) binding a homotrimer of TNF-alpha (tan). Certolizumab can block TNF in both its soluble form (freely circulating in the bloodstream) and its transmembrane form (bound to the membrane of a cell). From PDB: 5WUX.[11]
Certolizumab pegol is a monoclonal antibody directed against tumor necrosis factor alpha. More precisely, it is a PEGylated Fab' fragment of a humanized TNF inhibitor monoclonal antibody.[12]
Clinical trials
- Crohn's disease
- Positive results have been demonstrated in two phase III trials (PRECiSE 1 and 2) of certolizumab pegol versus placebo in moderate to severe active Crohn's disease.[1][12][13][14]
- Axial spondyloarthritis
- In 2013, a phase 3 double blind randomized placebo-controlled study found significantly positive results in patient self-reported questionnaires, with rapid improvement of function and pain reduction, in patients with axial spondyloarthritis.[15]
- Rheumatoid arthritis
- Certolizumab appears beneficial in those with rheumatoid arthritis.[16]
References
- ↑ 1.0 1.1 "Certolizumab pegol for the treatment of Crohn's disease". The New England Journal of Medicine 357 (3): 228–238. July 2007. doi:10.1056/NEJMoa067594. PMID 17634458.
- ↑ "Certolizumab pegol". mAbs 2 (2): 137–147. 2010. doi:10.4161/mabs.2.2.11271. PMID 20190560.
- ↑ "CDP-870 (certolizumab) in rheumatoid arthritis". Expert Opinion on Biological Therapy 5 (4): 601–606. April 2005. doi:10.1517/14712598.5.4.601. PMID 15934837.
- ↑ 4.0 4.1 "Drug Approval Package: Cimzia (Certolizumab Pegol) NDA #125160". December 24, 1999. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/125160s000TOC2.cfm.
- ↑ "Cimzia- certolizumab pegol kit Cimzia- certolizumab pegol injection, solution". April 24, 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b4c2c9dc-a0bb-4d64-a667-a67ebe88392d.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "Cimzia Approved in the US for the Treatment of Moderate to Severe Crohn's Disease". UCB press release. http://www.ucb.com/media-room/newsdetail/?det=1314787.
- ↑ "FDA Approvals: Patanase, Actonel, Cimzia". Medscape. May 1, 2008. http://www.medscape.com/viewarticle/573833.
- ↑ "Cimzia European Public Assessment Report". European Medicines Agency. http://www.emea.europa.eu/humandocs/Humans/EPAR/cimzia/cimzia.htm.
- ↑ "Cimzia (certolizumab pegol) approved by the U.S. FDA for treatment of adult patients with active psoriatic arthritis". http://www.ucb.presscentre.com/News/Cimzia-certolizumab-pegol-approved-by-the-U-S-FDA-for-treatment-of-adult-patients-with-active-ps-45c.aspx.
- ↑ "Molecular Basis for the Neutralization of Tumor Necrosis Factor α by Certolizumab Pegol in the Treatment of Inflammatory Autoimmune Diseases". International Journal of Molecular Sciences 18 (1): 228. January 2017. doi:10.3390/ijms18010228. PMID 28124979.
- ↑ 12.0 12.1 "Certolizumab pegol, a humanised anti-TNF pegylated FAb' fragment, is safe and effective in the maintenance of response and remission following induction in active Crohn's disease: a phase 3 study (precise)". Gut 54 (suppl 7): A82. 2005.
- ↑ "Certolizumab pegol administered subcutaneously is effective and well tolerated in patients with active Crohn's disease: results from a 26-week, placebo-controlled Phase 3 study (PRECiSE 1)". Gastroenterology 130 (4): A107. 2006.
- ↑ "New Analysis Shows Cimzia (Certolizumab Pegol) Maintained Remission and Response in Recent Onset Crohn's Disease" (Press release). UCB. October 23, 2006. Archived from the original on March 29, 2020. Retrieved November 15, 2009.
- ↑ "PMS50 – Rapid Improvements In Patient-Reported Outcomes With Certolizumab Pegol In Patients With Axial Spondyloarthritis, Including Ankylosing Spondylitis And Non-Radiographic Axial Spondyloarthritis: 24-Week Results Of A Phase 3 Double Blind Randomized Placebo-Controlled Study". Value in Health 16 (3): A227. May 2013. doi:10.1016/j.jval.2013.03.1150.
- ↑ "Certolizumab pegol (CDP870) for rheumatoid arthritis in adults". The Cochrane Database of Systematic Reviews 2017 (9): CD007649. September 2017. doi:10.1002/14651858.CD007649.pub4. PMID 28884785.
External links
- certolizumab+pegol at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

