Chemistry:Homodihydrocapsaicin
From HandWiki
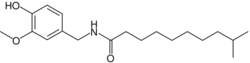
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-[(4-Hydroxy-3-methoxyphenyl)methyl]-9-methyldecanamide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H31NO3 | |
| Molar mass | 321.461 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
| Homodihydrocapsaicin | |
|---|---|
| Heat | Above peak |
| Scoville scale | 8,600,000[1] SHU |
Homodihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (Capsicum). Like capsaicin it is an irritant. Homodihydrocapsaicin accounts for about 1% of the total capsaicinoids mixture[2] and has about half the pungency of capsaicin. Pure homodihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It produces "numbing burn" in the throat and is one of the most prolonged and difficult to rinse out. On the Scoville scale it has 8,600,000 SHU (Scoville heat units).[1]
See also
- Capsaicin
- Dihydrocapsaicin
- Nordihydrocapsaicin
- Homocapsaicin
- Nonivamide
- Scoville scale
- Pepper spray
- Spice
References
- ↑ 1.0 1.1 "Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences". Critical Reviews in Food Science and Nutrition 29 (6): 435–474. 1991. doi:10.1080/10408399109527536. PMID 2039598.
- ↑ "Constitution and biosynthesis of capsaicin". J. Chem. Soc. C: 442. 1968. doi:10.1039/j39680000442.
External links
 |
