Short description: Chemical compound
Nordihydrocapsaicin
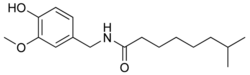
|
| Names
|
Preferred IUPAC name
N-[(4-Hydroxy-3-methoxyphenyl)methyl]-7-methyloctanamide |
| Other names
N-Vanillyl-7-methyloctanamide; Vanillylamide of 7-methyloctanoic acid; NDHC
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
|
|
|
| UNII
|
|
InChI=1S/C17H27NO3/c1-13(2)7-5-4-6-8-17(20)18-12-14-9-10-15(19)16(11-14)21-3/h9-11,13,19H,4-8,12H2,1-3H3,(H,18,20)  N NKey: VQEONGKQWIFHMN-UHFFFAOYSA-N  N NInChI=1/C17H27NO3/c1-13(2)7-5-4-6-8-17(20)18-12-14-9-10-15(19)16(11-14)21-3/h9-11,13,19H,4-8,12H2,1-3H3,(H,18,20) Key: VQEONGKQWIFHMN-UHFFFAOYAS
|
CC(C)CCCCCC(=O)NCC1=CC(=C(C=C1)O)OC
|
| Properties
|
|
|
C17H27NO3
|
| Molar mass
|
293.407 g·mol−1
|
|
|
Negligible
|
| Solubility
|
Soluble in DMSO, chloroform
|
| Hazards
|
| GHS pictograms
|
 
|
|
|
H300, H315, H319, H335
|
|
|
P264, P270, P280, P321, P330, P302+352, P362+364Script error: No such module "Preview warning".Category:GHS errors, P305+351+338, P405, P501
|
| NFPA 704 (fire diamond)
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?)
|
| Infobox references
|
|
|
|
Tracking categories (test):
| Nordihydrocapsaicin |
|---|
| Heat | Above peak
(pure Nordihydrocapsaicin is highly toxic) |
|---|
| Scoville scale | 9,100,000[1] SHU |
|---|
Nordihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (Capsicum).
Properties
Like capsaicin, it is an irritant. Nordihydrocapsaicin accounts for about 7% of the total capsaicinoids mixture[2] and has about half the pungency of capsaicin. Pure nordihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy solid. On the Scoville scale it has 9,100,000 SHU (Scoville heat units),[1] significantly higher than pepper spray.
See also
References
- ↑ 1.0 1.1 "Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences". Critical Reviews in Food Science and Nutrition 29 (6): 435–474. 1991. doi:10.1080/10408399109527536. PMID 2039598.
- ↑ "Constitution and biosynthesis of capsaicin". J. Chem. Soc. C: 442. 1968. doi:10.1039/j39680000442.
|
|---|
| TRPA | | Activators |
- 4-Hydroxynonenal
- 4-Oxo-2-nonenal
- 4,5-EET
- 12S-HpETE
- 15-Deoxy-Δ12,14-prostaglandin J2
- α-Sanshool (ginger, Sichuan and melegueta peppers)
- Acrolein
- Allicin (garlic)
- Allyl isothiocyanate (mustard, radish, horseradish, wasabi)
- AM404
- Bradykinin
- Cannabichromene (cannabis)
- Cannabidiol (cannabis)
- Cannabigerol (cannabis)
- Cinnamaldehyde (cinnamon)
- CR gas (dibenzoxazepine; DBO)
- CS gas (2-chlorobenzal malononitrile)
- Curcumin (turmeric)
- Dehydroligustilide (celery)
- Diallyl disulfide
- Dicentrine (Lindera spp.)
- Farnesyl thiosalicylic acid
- Formalin
- Gingerols (ginger)
- Hepoxilin A3
- Hepoxilin B3
- Hydrogen peroxide
- Icilin
- Isothiocyanate
- Ligustilide (celery, Angelica acutiloba)
- Linalool (Sichuan pepper, thyme)
- Methylglyoxal
- Methyl salicylate (wintergreen)
- N-Methylmaleimide
- Nicotine (tobacco)
- Oleocanthal (olive oil)
- Paclitaxel (Pacific yew)
- Paracetamol (acetaminophen)
- PF-4840154
- Phenacyl chloride
- Polygodial (Dorrigo pepper)
- Shogaols (ginger, Sichuan and melegueta peppers)
- Tear gases
- Tetrahydrocannabinol (cannabis)
- Thiopropanal S-oxide (onion)
- Umbellulone (Umbellularia californica)
- WIN 55,212-2
|
|---|
| Blockers | |
|---|
|
|---|
| TRPC | |
|---|
| TRPM | |
|---|
| TRPML | |
|---|
| TRPP | |
|---|
| TRPV | | Activators |
- 2-APB
- 5',6'-EET
- 9-HODE
- 9-oxoODE
- 12S-HETE
- 12S-HpETE
- 13-HODE
- 13-oxoODE
- 20-HETE
- α-Sanshool (ginger, Sichuan and melegueta peppers)
- Allicin (garlic)
- AM404
- Anandamide
- Bisandrographolide (Andrographis paniculata)
- Camphor (camphor laurel, rosemary, camphorweed, African blue basil, camphor basil)<!--TRPV1 and TRPV3-->
- Cannabidiol (cannabis)
- Cannabidivarin (cannabis)
- Capsaicin (chili pepper)
- Carvacrol (oregano, thyme, pepperwort, wild bergamot, others)
- DHEA
- Diacyl glycerol
- Dihydrocapsaicin (chili pepper)
- Estradiol
- Eugenol (basil, clove)
- Evodiamine (Euodia ruticarpa)
- Gingerols (ginger)
- GSK1016790A
- Heat
- Hepoxilin A3
- Hepoxilin B3
- Homocapsaicin (chili pepper)
- Homodihydrocapsaicin (chili pepper)
- Incensole (incense)
- Lysophosphatidic acid
- Low pH (acidic conditions)
- Menthol (mint)
- N-Arachidonoyl dopamine
- N-Oleoyldopamine
- N-Oleoylethanolamide
- Nonivamide (PAVA) (PAVA spray)
- Nordihydrocapsaicin (chili pepper)
- Paclitaxel (Pacific yew)
- Paracetamol (acetaminophen)
- Phorbol esters (e.g., 4α-PDD)
- Piperine (black pepper, long pepper)
- Polygodial (Dorrigo pepper)
- Probenecid
- Protons
- RhTx
- Rutamarin (Ruta graveolens)
- Resiniferatoxin (RTX) (Euphorbia resinifera/pooissonii)
- Shogaols (ginger, Sichuan and melegueta peppers)
- Tetrahydrocannabivarin (cannabis)
- Thymol (thyme, oregano)
- Tinyatoxin (Euphorbia resinifera/pooissonii)
- Tramadol
- Vanillin (vanilla)
- Zucapsaicin
|
|---|
| Blockers | |
|---|
|
|---|
|
 | Original source: https://en.wikipedia.org/wiki/Nordihydrocapsaicin. Read more |






