Chemistry:Cannabigerol
Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis.[1][2][3][4] It is one of more than 120 identified cannabinoids found in the plant genus Cannabis.[5][6] The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.[2][7]
During plant growth, most of the CBG is converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD), leaving about 1% CBG in finished plant material.[8] Some strains, however, produce larger amounts of CBG and CBGA, while having low quantities of other cannabinoids, like THC and CBD.[9]
The pharmacodynamics of CBG are complex.[1][2][3] It is a relatively weak ligand of the cannabinoid receptors, where it acts as a weak partial agonist.[1][2][3] Conversely, it is a much more potent agonist of the α2-adrenergic receptor, antagonist of the serotonin 5-HT1A receptor, and antagonist of the transient receptor potential channel TRPM8.[1][2][3] CBG also has a variety of other actions that may additionally contribute to its effects.[1][2][3]
CBG is sold as a dietary supplement.[2] Safety concerns have been raised due to the potent activation of α2-adrenergic receptors by CBG, which may produce sedation and potentially undesirable cardiovascular effects such as decreased heart rate and blood pressure.[2]
Pharmacology
This section needs additional citations for verification. (December 2024) (Learn how and when to remove this template message) |
Pharmacodynamics
In vitro, CBG has identified pharmacodynamic actions and its mechanism of action appears to be from interactions with multiple targets.[1][2][3]
CBG is a weak ligand of the cannabinoid CB1 and CB2 receptors with affinities (Ki) of 380–2,600 nM and 153–3,460 nM, respectively.[1][2][10][11][12] It is a weak partial agonist or antagonist of both of these receptors.[1][2][3] There is no information on the binding or activity of CBG at the GPR55 (the potential non-homologous CB3 receptor).[2][3] CBG has relatively low affinity for the cannabinoid receptors, with approximately 5-fold lower affinity for the CB1 receptor and 27-fold lower affinity for the CB2 receptor than THC.[citation needed]
CBG is a highly potent agonist of the α2-adrenergic receptor (EC50 = 0.2–72.8 nM) and a moderately potent antagonist of the serotonin 5-HT1A receptor (KB = 51.9 nM).[2][1][13] Activation of the α2-adrenergic receptor by CBG might produce effects including sedation, dry mouth, and decreased heart rate and blood pressure.[2] This has raised safety concerns about CBG.[2] The actions of CBG at the α2-adrenergic receptor and 5-HT1A receptor are of far greater potency than its interactions with the cannabinoid receptors and are more likely to be involved in its pharmacodynamic effects.[citation needed]
The compound is a weak agonist of the transient receptor potential channels TRPA1 (EC50 = 700 nM), TRPV1 (EC50 = 1,300 nM), TRPV2 (EC50 = 1,720 nM), TRPV3 (EC50 = 1,000 nM), and TRPV4 (EC50 = 5,100 nM) (efficacy 18–100% at these targets) and a more potent antagonist of the transient receptor potential channel TRPM8 (IC50 = 160 nM).[10][2][1][11][3] It is also a weak agonist of the peroxisome proliferator-activated receptor PPAR-γ (EC50 = 1,270–15,700 nM).[2][1][11]
CBG is a voltage-gated sodium channel (VGSC) blocker (Nav1.1, Nav1.2, Nav1.5, and Nav1.7) and voltage-dependent calcium channel (VDCC) blocker.[3][1][14] Inhibition of VGSCs may be involved in the analgesic effects of CBG.[3]
It shows no inhibition of several endocannabinoid-metabolizing enzymes including fatty acid amide hydrolase (FAAH), diacylglycerol lipase (DGL), and N-acylethanolamine acid amide hydrolase (NAAA).[11] However, other research has found that CBG does inhibit FAAH and DGL, as well as monoacylglycerol lipase (MAGL), although it is less potent as an inhibitor of FAAH than cannabidiol (CBD). Aside from endocannabinoid-metabolizing enzymes, CBG is a weak inhibitor of the cyclooxygenase COX-1 and COX-2 enzymes (30% inhibition of each at 25,000 nM).[1] In addition, it has been found to inhibit both the metabolism and reuptake of anandamide.[1]
Pharmacokinetics
The pharmacokinetics of CBG have been studied in animals and to a lesser extent in humans.[1] CBG is metabolized in the liver by CYP2J2, similarly to other cannabinoids as well as endocannabinoids.[1]
Chemistry
CBG is a highly lipophilic and hydrophobic compound.[3] Its predicted log P ranges from 7.0 to 7.5.[7][15][16]
Synthetic derivatives of CBG have been synthesized and studied.[1]
History
CBG was isolated from cannabis in 1964.
Society and culture
Legal status
CBG is not scheduled by the United Nations Convention on Psychotropic Substances. In the United States, CBG derived from marijuana is illegal under the Controlled Substances Act, while CBG derived from hemp is legal, as long as the hemp THC content is less than 0.3% of dry weight.[17][18]
In Switzerland, it is legal to produce hemp rich in CBG as a tobacco substitute, as long as its THC content remains below 1.0%.[19]
Regulation
As of 2022, the US Food and Drug Administration has issued numerous warning letters to American companies for illegally marketing cannabis supplement products,[17] including one selling CBG products with unproven illegal claims of efficacy against the COVID-19 virus and inflammation.[20]
Biosynthesis
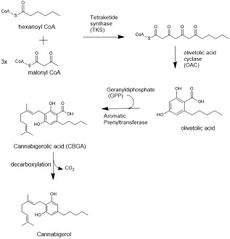
The biosynthesis of CBG begins by loading hexanoyl-CoA onto a polyketide synthase assembly protein and subsequent condensation with three molecules of malonyl-CoA.[21] This polyketide is cyclized to olivetolic acid via olivetolic acid cyclase, and then prenylated with a ten carbon isoprenoid precursor, geranyl pyrophosphate, using an aromatic prenyltransferase enzyme, geranyl-pyrophosphate—olivetolic acid geranyltransferase, to biosynthesize cannabigerolic acid, which can then be decarboxylated to yield CBG.[2][4]
Research
CBG is under laboratory research to determine its pharmacological properties and potential effects in disease conditions, with no conclusions about therapeutic effects or safety, as of 2021.[2][12][22] A clinical trial published in July 2024 assessed the effects of CBG on anxiety, stress, and mood.[23][24] CBG has been determined to have antimicrobial properties both in in vivo and in vitro models.[25] The clinical development has, however, been hindered by its poor drug metabolism and pharmacokinetic properties.[26]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 "Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives". Evid Based Complement Alternat Med 2022. 2022. doi:10.1155/2022/3336516. PMID 36397993.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 "The pharmacological case for cannabigerol". The Journal of Pharmacology and Experimental Therapeutics 376 (2): 204–212. 2021. doi:10.1124/jpet.120.000340. ISSN 0022-3565. PMID 33168643. https://jpet.aspetjournals.org/content/376/2/204.long.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 "Non-psychotropic phytocannabinoid interactions with voltage-gated sodium channels: An update on cannabidiol and cannabigerol". Front Physiol 13. 2022. doi:10.3389/fphys.2022.1066455. PMID 36439273.
- ↑ 4.0 4.1 "An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol". Frontiers in Pharmacology 8. 2017. doi:10.3389/fphar.2017.00422. PMID 28701957.
- ↑ "Phytochemistry of Cannabis sativa L". Phytochemistry of Cannabis sativa L.. Progress in the Chemistry of Organic Natural Products. 103. 2017. pp. 1–36. doi:10.1007/978-3-319-45541-9_1. ISBN 978-3-319-45539-6.
- ↑ "Molecular Pharmacology of Phytocannabinoids". Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. 103. 2017. pp. 61–101. doi:10.1007/978-3-319-45541-9_3. ISBN 978-3-319-45539-6.
- ↑ 7.0 7.1 "Cannabigerol; ID 5315659". PubChem, National Library of Medicine, US National Institutes of Health. 2 July 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Cannabigerol.
- ↑ "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products 79 (2): 324–331. February 2016. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472. Bibcode: 2016JNAtP..79..324A. https://figshare.com/articles/journal_contribution/5028338.
- ↑ "Cannabigerol and cannabichromene in Cannabis sativa L." (in en). Acta Pharmaceutica 71 (3): 355–364. 2021-09-01. doi:10.2478/acph-2021-0021. PMID 36654096.
- ↑ 10.0 10.1 "Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the "Big Two"". J Nat Prod 84 (1): 142–160. January 2021. doi:10.1021/acs.jnatprod.0c00965. PMID 33356248. Bibcode: 2021JNAtP..84..142S.
- ↑ 11.0 11.1 11.2 11.3 Liu, Tiqing. "BindingDB BDBM50318487 CHEMBL497318::Cannabigerol". https://www.bindingdb.org/bind/chemsearch/marvin/MolStructure.jsp?monomerid=50318487.
- ↑ 12.0 12.1 "Molecular Targets of the Phytocannabinoids: A Complex Picture". Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. 103. 2017. pp. 103–131. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6.
- ↑ "Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist". Br J Pharmacol 159 (1): 129–141. January 2010. doi:10.1111/j.1476-5381.2009.00515.x. PMID 20002104.
- ↑ "Inhibition of sodium conductance by cannabigerol contributes to a reduction of dorsal root ganglion neuron excitability". Br J Pharmacol 179 (15): 4010–4030. August 2022. doi:10.1111/bph.15833. PMID 35297036.
- ↑ "Cannabigerol: Uses, Interactions, Mechanism of Action". 1 February 2019. https://go.drugbank.com/drugs/DB14734.
- ↑ "Cannabigerol". 14 August 2024. https://www.chemspider.com/Chemical-Structure.4474921.html.
- ↑ 17.0 17.1 "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)". US Food and Drug Administration. 21 January 2021. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd.
- ↑ "USC > Title 21 > Chapter 13 > Subchapter I > Part A > § 802. Definitions: (16)". 2016. https://www.gpo.gov/fdsys/pkg/USCODE-2016-title21/pdf/USCODE-2016-title21-chap13-subchapI-partA-sec802.pdf.
- ↑ BAG, Bundesamt für Gesundheit. "Häufig gestellte Fragen (FAQ) zu Tabakersatzprodukten mit THC-armem Hanf mit CBD" (in de). https://www.bag.admin.ch/bag/de/home/gesetze-und-bewilligungen/gesuche-bewilligungen/gesuche-bewilligungen-im-bereich-sucht/gesetzliche-vorgaben-tabakprodukte/faq-cbd.html.
- ↑ "Warning Letter to Greenway Herbal Products LLC; Ref. 627042". Office of Compliance, Center for Drug Evaluation and Research, Food and Drug Administration. 28 March 2022. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/greenway-herbal-products-llc-627042-03282022.
- ↑ "Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides". Proceedings of the National Academy of Sciences of the United States of America 109 (31): 12811–12816. July 2012. doi:10.1073/pnas.1200330109. PMID 22802619. Bibcode: 2012PNAS..10912811G.
- ↑ "The Use of Cannabinoids in Colitis: A Systematic Review and Meta-Analysis". Inflammatory Bowel Diseases 24 (4): 680–697. March 2018. doi:10.1093/ibd/izy014. PMID 29562280.
- ↑ "Acute effects of cannabigerol on anxiety, stress, and mood: a double-blind, placebo-controlled, crossover, field trial". Sci Rep 14 (1). July 2024. doi:10.1038/s41598-024-66879-0. PMID 39003387. Bibcode: 2024NatSR..1416163C.
- ↑ "Cannabigerol (CBG) Reduces Anxiety and Improves Memory". 31 July 2024. https://neurosciencenews.com/cbg-anxiety-memory-27507/.
- ↑ "Uncovering the Hidden Antibiotic Potential of Cannabis". ACS Infectious Diseases 6 (3): 338–346. March 2020. doi:10.1021/acsinfecdis.9b00419. PMID 32017534.
- ↑ "Plant Antibacterials: The Challenges and Opportunities". Heliyon 10 (10). 2024. doi:10.1016/j.heliyon.2024.e31145.
 |
