Chemistry:Stevioside
| |
| Names | |
|---|---|
| IUPAC name
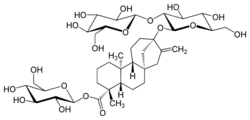
β-D-Glucopyranosyl 13-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyloxy]-5β,8α,9β,10α,13α-kaur-16-en-18-oate
| |
| Systematic IUPAC name
(4R,4aS,6aR,9S,11aR,11bS)-9-{[(2S,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-4,11b-dimethyl-8-methylidenetetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C38H60O18 | |
| Molar mass | 804.8722 |
| Appearance | white powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Stevioside is a glycoside derived from the stevia plant, which can be used as a sweetener.[1] Evidence of benefit is lacking for long-term effects on weight loss and heart disease risks.[2]
Origin
Stevioside is the main sweetener (along with rebaudioside A) found in the leaves of Stevia rebaudiana, a plant originating in South America. Dried leaves, as well as aqueous extracts, have been used for decades as a sweetener in many countries, notably in Latin America and Asia (Japan, China).[3] Stevioside was discovered in 1931 by French chemists who gave it its name.[3] The sweetening power of stevioside was estimated to be about 300 times stronger than cane sugar.[3]
Safety
Since 2008, the U.S. Food and Drug Administration has not objected to the use of stevia extracts and some purified steviosides, mainly stevioside and rebaudioside, as GRAS for safe use as an ingredient in manufactured foods.[4]
See also
References
- ↑ "Steviol glycosides: chemical diversity, metabolism, and function". Journal of Natural Products 76 (6): 1201–28. June 2013. doi:10.1021/np400203b. PMID 23713723.
- ↑ "Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies". CMAJ 189 (28): E929–E939. July 2017. doi:10.1503/cmaj.161390. PMID 28716847.
- ↑ 3.0 3.1 3.2 Scientific Committee on Food (17 June 1999). "Opinion On Stevioside as a Sweetener". The European Commission. http://ec.europa.eu:80/food/fs/sc/scf/out34_en.pdf.
- ↑ Perrier, Judith D.; Mihalov, Jeremy J.; Carlson, Susan J. (2018). "FDA regulatory approach to steviol glycosides". Food and Chemical Toxicology 122: 132–142. doi:10.1016/j.fct.2018.09.062. ISSN 0278-6915. PMID 30268795.
 |
