Icilin
|
| Names
|
Preferred IUPAC name
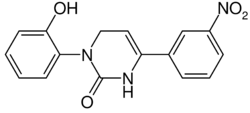
3-(2-Hydroxyphenyl)-6-(3-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one |
| Other names
1-(2-Hydroxyphenyl)-4-(3-nitrophenyl)-3,6-dihydropyrimidin-2-one
AG-3-5
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
|
|
|
|
|
|
| UNII
|
|
InChI=1S/C16H13N3O4/c20-15-7-2-1-6-14(15)18-9-8-13(17-16(18)21)11-4-3-5-12(10-11)19(22)23/h1-8,10,20H,9H2,(H,17,21)  N NKey: RCEFMOGVOYEGJN-UHFFFAOYSA-N  N NInChI=1/C16H13N3O4/c20-15-7-2-1-6-14(15)18-9-8-13(17-16(18)21)11-4-3-5-12(10-11)19(22)23/h1-8,10,20H,9H2,(H,17,21) Key: RCEFMOGVOYEGJN-UHFFFAOYAQ
|
O=C3N(c1c(O)cccc1)C/C=C(/c2cccc([N+]([O-])=O)c2)N3
|
| Properties
|
|
|
C16H13N3O4
|
| Molar mass
|
311.29 g/mol
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?)
|
| Infobox references
|
|
|
|
Tracking categories (test):
Icilin (AG-3-5) is a synthetic super-agonist of the transient receptor potential M8 (TRPM8) ion channel. Although structurally not related to menthol, it produces an extreme sensation of cold, both in humans and animals. It is almost 200 times more potent than menthol, and 2.5 times more efficacious.[1] Despite their similar effects, icilin activates the TRPM8 receptor in a different way than menthol does.[2] Icilin has been shown to be effective in the treatment of pruritus in an experimental model of itch.[3] It is now used as a research tool for the study of TRP channels, although despite its high potency it is less selective for TRPM8 over other related ion channels than are other compounds such as WS-12.
References
External links
|
|---|
| TRPA | | Activators |
- 4-Hydroxynonenal
- 4-Oxo-2-nonenal
- 4,5-EET
- 12S-HpETE
- 15-Deoxy-Δ12,14-prostaglandin J2
- α-Sanshool (ginger, Sichuan and melegueta peppers)
- Acrolein
- Allicin (garlic)
- Allyl isothiocyanate (mustard, radish, horseradish, wasabi)
- AM404
- Bradykinin
- Cannabichromene (cannabis)
- Cannabidiol (cannabis)
- Cannabigerol (cannabis)
- Cinnamaldehyde (cinnamon)
- CR gas (dibenzoxazepine; DBO)
- CS gas (2-chlorobenzal malononitrile)
- Curcumin (turmeric)
- Dehydroligustilide (celery)
- Diallyl disulfide
- Dicentrine (Lindera spp.)
- Farnesyl thiosalicylic acid
- Formalin
- Gingerols (ginger)
- Hepoxilin A3
- Hepoxilin B3
- Hydrogen peroxide
- Icilin
- Isothiocyanate
- Ligustilide (celery, Angelica acutiloba)
- Linalool (Sichuan pepper, thyme)
- Methylglyoxal
- Methyl salicylate (wintergreen)
- N-Methylmaleimide
- Nicotine (tobacco)
- Oleocanthal (olive oil)
- Paclitaxel (Pacific yew)
- Paracetamol (acetaminophen)
- PF-4840154
- Phenacyl chloride
- Polygodial (Dorrigo pepper)
- Shogaols (ginger, Sichuan and melegueta peppers)
- Tear gases
- Tetrahydrocannabinol (cannabis)
- Thiopropanal S-oxide (onion)
- Umbellulone (Umbellularia californica)
- WIN 55,212-2
|
|---|
| Blockers | |
|---|
|
|---|
| TRPC | |
|---|
| TRPM | |
|---|
| TRPML | |
|---|
| TRPP | |
|---|
| TRPV | | Activators |
- 2-APB
- 5',6'-EET
- 9-HODE
- 9-oxoODE
- 12S-HETE
- 12S-HpETE
- 13-HODE
- 13-oxoODE
- 20-HETE
- α-Sanshool (ginger, Sichuan and melegueta peppers)
- Allicin (garlic)
- AM404
- Anandamide
- Bisandrographolide (Andrographis paniculata)
- Camphor (camphor laurel, rosemary, camphorweed, African blue basil, camphor basil)<!--TRPV1 and TRPV3-->
- Cannabidiol (cannabis)
- Cannabidivarin (cannabis)
- Capsaicin (chili pepper)
- Carvacrol (oregano, thyme, pepperwort, wild bergamot, others)
- DHEA
- Diacyl glycerol
- Dihydrocapsaicin (chili pepper)
- Estradiol
- Eugenol (basil, clove)
- Evodiamine (Euodia ruticarpa)
- Gingerols (ginger)
- GSK1016790A
- Heat
- Hepoxilin A3
- Hepoxilin B3
- Homocapsaicin (chili pepper)
- Homodihydrocapsaicin (chili pepper)
- Incensole (incense)
- Lysophosphatidic acid
- Low pH (acidic conditions)
- Menthol (mint)
- N-Arachidonoyl dopamine
- N-Oleoyldopamine
- N-Oleoylethanolamide
- Nonivamide (PAVA) (PAVA spray)
- Nordihydrocapsaicin (chili pepper)
- Paclitaxel (Pacific yew)
- Paracetamol (acetaminophen)
- Phorbol esters (e.g., 4α-PDD)
- Piperine (black pepper, long pepper)
- Polygodial (Dorrigo pepper)
- Probenecid
- Protons
- RhTx
- Rutamarin (Ruta graveolens)
- Resiniferatoxin (RTX) (Euphorbia resinifera/pooissonii)
- Shogaols (ginger, Sichuan and melegueta peppers)
- Tetrahydrocannabivarin (cannabis)
- Thymol (thyme, oregano)
- Tinyatoxin (Euphorbia resinifera/pooissonii)
- Tramadol
- Vanillin (vanilla)
- Zucapsaicin
|
|---|
| Blockers | |
|---|
|
|---|
|
 | Original source: https://en.wikipedia.org/wiki/Icilin. Read more |


