Chemistry:Neodymium(II) iodide
| |
| Names | |
|---|---|
| IUPAC name
Diiodoneodymium
| |
| Other names
Neodymium diiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| NdI2 | |
| Molar mass | 398.05 |
| Appearance | dark violet solid[1] |
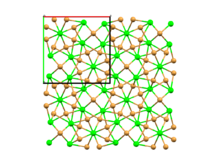
| Structure[2] | |
| SrBr2 type (Tetragonal) | |
| P4/n (No. 85) | |
a = 1257.3 pm, c = 765.8 pm
| |
Formula units (Z)
|
10 |
| Hazards | |
| GHS pictograms |  [3] [3]
|
| GHS Signal word | Warning[3] |
| Related compounds | |
Other anions
|
Neodymium(II) fluoride, Neodymium(II) chloride, Neodymium(II) bromide |
Other cations
|
lanthanum diiodide, cerium diiodide, praseodymium diiodide, europium diiodide, samarium(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.
Neodymium(II) iodide is a violet solid.[1] The compound is not stoichiometric.[4] It melts at 562°C.[5]
Preparation
Neodymium(II) iodide can be made by heating molten neodymium(III) iodide with neodymium metal at 800 and 580°C for 12 hours.[4] It can also be obtained by reducing neodymium(III) iodide with neodymium in a vacuum at 800 to 900°C:[1]
- Nd + 2NdI3 → 3NdI2
The reaction of neodymium with mercury(II) iodide is also possible because neodymium is more reactive than mercury:[1]
- Nd + HgI2 → NdI2 + Hg
Direct preparation from iodine and neodymium is also possible:[6]
- Nd + I2 → NdI2
The compound was first synthesized by John D. Corbett in 1961.[7]
Properties
Neodymium(II) iodide is a violet solid.[1] The compound is extremely hygroscopic, and can only be stored and handled under carefully dried inert gas or under a high vacuum.[8] In air it converts into hydrates by absorbing moisture, but these are unstable and more or less rapidly transform into oxide iodides with the evolution of hydrogen:
- 2NdI2 + 2H2O → 2NdOI + H2↑ + 2HI
Neodymium(II) iodide is not stoichiometric, and has a formula of closer to NdI1.95.[4] It melts at 562°C.[5] It has a strontium(II) bromide-type crystal structure.[1] Under pressure, this transforms into the molybdenum disilicide structure typically seen in intermetallic compound, which is already present under normal conditions in other rare earth diiodides (e.g. praseodymium(II) iodide and lanthanum(II) iodide).[9] It forms complexes with tetrahydrofuran and other organic compounds.[10][11][12]
Neodymium(II) iodide is an electrical insulator.[4]
Reactions
Neodymium(II) iodide reacts with organohalides by extracting the halogen, resulting in dimers, oligomers or reactions with the solvent.[12]
Solvates are known with tetrahydrofuran and dimethoxyethane: NdI2(THF)2 and NdI2(DME)2.[13]
Neodymium(II) iodide reduces hot nitrogen to form an iodide nitride: (NdI2)3N which with THF also gives (NdI)3N2.[14]
It reacts with cyclopentadiene in THF to give CpNdI2(THF)3.[15]
Applications
Neodymium(II) iodide can be used as a reducing agent or catalyst[16] in organic chemistry.[17]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 (in de) Handbuch der präparativen anorganischen Chemie. Stuttgart: Enke. 1975. p. 1081. ISBN 3-432-02328-6. OCLC 310719485.
- ↑ Beck, H. P. (1976-11-01). "Notizen: NdI2-II, eine metallisch leitende Hochdruckmodifikation ? / NdI2, a Metallic High Pressure Modification ?" (in de). Zeitschrift für Naturforschung B (Walter de Gruyter GmbH) 31 (11): 1548–1549. doi:10.1515/znb-1976-1128. ISSN 1865-7117.
- ↑ 3.0 3.1 See https://pubchem.ncbi.nlm.nih.gov/compound/Neodymium_II_-iodide
- ↑ 4.0 4.1 4.2 4.3 Sallach, Robert A.; Corbett, John D. (July 1964). "Magnetic Susceptibilities of Neodymium (II) Chloride and Iodide". Inorganic Chemistry 3 (7): 993–995. doi:10.1021/ic50017a015.
- ↑ 5.0 5.1 Druding, Leonard F.; Corbett, John D. (June 1961). "Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide 1". Journal of the American Chemical Society 83 (11): 2462–2467. doi:10.1021/ja01472a010.
- ↑ Karl A. Jr. Gschneidner, Jean-Claude Bunzli, Vitalij K. Pecharsky (2009). Handbook on the Physics and Chemistry of Rare Earths. Elsevier. p. 247. ISBN 978-008093257-6. https://books.google.com/books?id=CV-YrFC8_YIC&pg=PA247.
- ↑ Angelika Jungmann, R. Claessen, R. Zimmermann, G. e. Meng, P. Steiner, S. Hüfner, S. Tratzky, K. Stöwe, H. P. Beck: Photoemission of LaI2 and CeI2. In: Zeitschrift für Physik B Condensed Matter. 97, 1995, S. 25–34, doi:10.1007/BF01317584.
- ↑ Ortu, Fabrizio (2022). "Rare Earth Starting Materials and Methodologies for Synthetic Chemistry". Chem. Rev. 122 (6): 6040–6116. doi:10.1021/acs.chemrev.1c00842. PMID 35099940.
- ↑ Ralf Alsfasser, Erwin Riedel (2007). Moderne Anorganische Chemie. Walter de Gruyter. p. 188. ISBN 978-311019060-1. https://books.google.com/books?id=HwY4be5bH_sC&pg=PA188.
- ↑ Mikhail N. Bochkarev, Igor L. Fedushkin, Sebastian Dechert, Anatolii A. Fagin, Herbert Schumann: [NdI2(thf)5], der erste kristallographisch charakterisierte Neodym(II)-Komplex. In: Angewandte Chemie. 113, 2001, S. 3268–3270, doi:10.1002/1521-3757(20010903)113:17<3268::AID-ANGE3268>3.0.CO;2-K.
- ↑ G. V. Khoroshen kov, A. A. Fagin, M. N. Bochkarev, S. Dechert, H. Schumann: Reactions of neodymium(II), dysprosium(II), and thulium(II) diiodides with cyclopentadiene In: Russian Chemical Bulletin. 52, S. 1715–1719, doi:10.1023/A:1026132017155.
- ↑ 12.0 12.1 Fagin, Anatolii A.; Balashova, Tatyana V.; Kusyaev, Dmitrii M. et al. (March 2006). "Reactions of neodymium(II) iodide with organohalides". Polyhedron 25 (5): 1105–1110. doi:10.1016/j.poly.2005.08.050.
- ↑ Bochkarev, Mikhail N.; Fagin, Anatolii A. (24 September 1999). "A New Route to Neodymium(II) and Dysprosium(II) Iodides". Chemistry - A European Journal 5 (10): 2990–2992. doi:10.1002/(SICI)1521-3765(19991001)5:10<2990::AID-CHEM2990>3.0.CO;2-U.
- ↑ Fagin, A. A.; Salmova, S. V.; Bochkarev, M. N. (January 2009). "Reduction of nitrogen with neodymium(II) and dysprosium(II) diiodides and selected properties of the resulting nitrides". Russian Chemical Bulletin 58 (1): 230–233. doi:10.1007/s11172-009-0034-2.
- ↑ Khoroshen'kov, G. V.; Fag, A. A.; Bochkarev, M. N.; Dechert, S.; Schumann, H. (1 August 2003). "Reactions of neodymium(ii), dysprosium(ii), and thulium(ii) diiodides with cyclopentadiene. Molecular structures of complexes CpTmI2(THF)3 and [NdI2(THF)5]+[NdI4(THF)2]–". Russian Chemical Bulletin 52 (8): 1715–1719. doi:10.1023/A:1026132017155.
- ↑ Fundamental Chemistry (2006). Neodymium Based Ziegler Catalysts. Springer. p. 13. ISBN 354034809-3. https://books.google.com/books?id=xX9mYOp2HFYC&pg=PA13.
- ↑ Handbook on the Physics and Chemistry of Rare Earths. Elsevier. 2009. p. 261. ISBN 978-008093257-6. https://books.google.com/books?id=CV-YrFC8_YIC&pg=PA261.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |
