Chemistry:Neodymium(III) chloride
| |||
| Names | |||
|---|---|---|---|
| Other names
Neodymium trichloride
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| NdCl3, NdCl3·6H2O (hydrate) | |||
| Molar mass | 250.598 g/mol | ||
| Appearance | Mauve-colored powder[1] hygroscopic | ||
| Density | 4.13 g/cm3 (2.3 for hydrate)[1] | ||
| Melting point | 759 °C (1,398 °F; 1,032 K)[1] | ||
| Boiling point | 1,600 °C (2,910 °F; 1,870 K)[1] | ||
| 1 kg/L at 25 °C[1] | |||
| Solubility in ethanol | 0.445 kg/L | ||
| Structure[2] | |||
| hexagonal (UCl3 type), hP8 | |||
| P63/m, No. 176 | |||
a = 0.73988 nm, c = 0.42423 nm
| |||
Formula units (Z)
|
2 | ||
| Tricapped trigonal prismatic (nine-coordinate) | |||
| Hazards | |||
| Safety data sheet | External SDS | ||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions
|
Neodymium(III) bromide Neodymium(III) oxide | ||
Other cations
|
LaCl3, SmCl3, PrCl3, EuCl3, CeCl3, GdCl3, TbCl3, Promethium(III) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
Appearance

NdCl3 is a mauve colored hygroscopic solid whose color changes to purple upon absorption of atmospheric water. The resulting hydrate, like many other neodymium salts, has the interesting property that it appears different colors under fluorescent light- In the chloride's case, light yellow (see picture).[3]
Structure
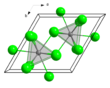
Solid
The anhydrous NdCl3 features Nd in a nine-coordinate tricapped trigonal prismatic geometry and crystallizes with the UCl3 structure. This hexagonal structure is common for many halogenated lanthanides and actinides such as LaCl3, LaBr3, SmCl3, PrCl3, EuCl3, CeCl3, CeBr3, GdCl3, AmCl3 and TbCl3 but not for YbCl3 and LuCl3.[4]
Solution
The structure of neodymium(III) chloride in solution crucially depends on the solvent: In water, the major species are Nd(H2O)83+, and this situation is common for most rare earth chlorides and bromides. In methanol, the species are NdCl2(CH3OH)6+ and in hydrochloric acid NdCl(H2O)72+. The coordination of neodymium is octahedral (8-fold) in all cases, but the ligand structure is different.[5]
Properties
NdCl3 is a soft paramagnetic solid, which turns ferromagnetic at very low temperature of 0.5 K.[6] Its electrical conductivity is about 240 S/m and heat capacity is ~100 J/(mol·K).[7] NdCl3 is readily soluble in water and ethanol, but not in chloroform or ether. Reduction of NdCl3 with Nd metal at temperatures above 650 °C yields NdCl2:[8]
- 2 NdCl3 + Nd → 3 NdCl2
Heating of NdCl3 with water vapors or silica produces neodymium oxochloride:
- NdCl3 + H2O → NdOCl + 2 HCl
- 2 NdCl3 + SiO2 → 2 NdOCl + SiCl4
Reacting NdCl3 with hydrogen sulfide at about 1100 °C produces neodymium sulfide:
- 2 NdCl3 + 3 H2S → 2 Nd2S3 + 6 HCl
Reactions with ammonia and phosphine at high temperatures yield neodymium nitride and phosphide, respectively:
- NdCl3 + NH3 → NdN + 3 HCl
- NdCl3 + PH3 → NdP + 3 HCl
Whereas the addition of hydrofluoric acid produces neodymium fluoride:[9]
- NdCl3 + 3 HF → NdF3 + 3 HCl
Preparation

NdCl3 is produced from minerals monazite and bastnäsite. The synthesis is complex because of the low abundance of neodymium in the Earth's crust (38 mg/kg) and because of difficulty of separating neodymium from other lanthanides. The process is however easier for neodymium than for other lanthanides because of its relatively high content in the mineral – up to 16% by weight, which is the third highest after cerium and lanthanum.[10] Many synthesis varieties exist and one can be simplified as follows:
The crushed mineral is treated with hot concentrated sulfuric acid to produce water-soluble sulfates of rare earths. The acidic filtrates are partially neutralized with sodium hydroxide to pH 3–4. Thorium precipitates out of solution as hydroxide and is removed. After that the solution is treated with ammonium oxalate to convert rare earths into their insoluble oxalates. The oxalates are converted to oxides by annealing. The oxides are dissolved in nitric acid that excludes the main components, cerium, whose oxide is insoluble in HNO3. Neodymium oxide is separated from other rare-earth oxides by ion exchange. In this process, rare-earth ions are adsorbed onto suitable resin by ion exchange with hydrogen, ammonium or cupric ions present in the resin. The rare earth ions are then selectively washed out by suitable complexing agent, such as ammonium citrate or nitrilotracetate.[9]
This process normally yields Nd2O3; the oxide is difficult to directly convert to elemental neodymium, which is often the goal of the whole technological procedure. Therefore, the oxide is treated with hydrochloric acid and ammonium chloride to produce the less stable NdCl3:[9]
- Nd2O3 + 6 NH4Cl → 2 NdCl3 + 3 H2O + 6 NH3
The thus produced NdCl3 quickly absorbs water and converts to NdCl3·6H2O hydrate, which is stable for storage, and can be converted back into NdCl3 when necessary. Simple rapid heating of the hydrate is not practical for that purpose because it causes hydrolysis with consequent production of Nd2O3.[11] Therefore, anhydrous NdCl3 is prepared by dehydration of the hydrate either by slowly heating to 400 °C with 4-6 equivalents of ammonium chloride under high vacuum, or by heating with an excess of thionyl chloride for several hours.[4][12][13][14] The NdCl3 can alternatively be prepared by reacting neodymium metal with hydrogen chloride or chlorine, though this method is not economical due to the relatively high price of the metal and is used for research purposes only. After preparation, it is usually purified by high temperature sublimation under high vacuum.[4][15][16]
Applications
Production of neodymium metal

Neodymium(III) chloride is the most common starting compound for production of neodymium metal. NdCl3 is heated with ammonium chloride or ammonium fluoride and hydrofluoric acid or with alkali or alkaline earth metals in vacuum or argon atmosphere at 300–400 °C.
- 2 NdCl3 + 3 Ca → 2 Nd + 3 CaCl2
An alternative route is electrolysis of molten mixture of anhydrous NdCl3 and NaCl, KCl, or LiCl at temperatures about 700 °C. The mixture melts at those temperatures, even though they are lower than the melting points of NdCl3 and KCl (~770 °C).[17]
Lasers and fiber amplifiers
Although NdCl3 itself does not have strong luminescence,[18] it serves as a source of Nd3+ ions for various light emitting materials. The latter include Nd-YAG lasers and Nd-doped optical fiber amplifiers, which amplify light emitted by other lasers. The Nd-YAG laser emits infrared light at 1.064 micrometres and is the most popular solid-state laser (i.e. laser based on a solid medium). The reason for using NdCl3 rather than metallic neodymium or its oxide, in fabrication of fibers is easy decomposition of NdCl3 during the chemical vapor deposition; the latter process is widely used for the fiber grows.[19]
Neodymium(III) chloride is a dopant not only of traditional silica-based optical fibers, but of plastic fibers (dopedphotolime-gelatin, polyimide, polyethylene, etc.) as well.[20] It is also used in as an additive into infrared organic light-emitting diodes.[21][22] Besides, neodymium doped organic films can not only act as LEDs, but also as color filters improving the LED emission spectrum.[23]
Solubility of neodymium(III) chloride (and other rare-earth salts) is various solvents results in a new type of rare-earth laser, which uses not a solid but liquid as an active medium. The liquid containing Nd3+ ions is prepared in the following reactions:
- SnCl4 + 2 SeOCl2 → SnCl62− + 2 SeOCl+
- SbCl5 + SeOCl2 → SbCl6− + SeOCl+
- 3 SeOCl+ + NdCl3 → Nd3+(solv) + 3 SeOCl2,
where Nd3+ is in fact the solvated ion with several selenium oxychloride molecules coordinated in the first coordination sphere, that is [Nd(SeOCl2)m]3+. The laser liquids prepared by this technique emits at the same wavelength of 1.064 micrometres and possess properties, such as high gain and sharpness of the emission, that are more characteristic of crystalline than Nd-glass lasers. The quantum efficiency of those liquid lasers was about 0.75 relative to the traditional Nd:YAG laser.[21]
Catalysis
Another important application of NdCl3 is in catalysis—in combination with organic chemicals, such as triethylaluminium and 2-propanol, it accelerates polymerization of various dienes. The products include such general purpose synthetic rubbers as polybutylene, polybutadiene, and polyisoprene.[11][24][25]
Neodymium(III) chloride is also used to modify titanium dioxide. The latter is one of the most popular inorganic photocatalyst for decomposition of phenol, various dyes and other waste water contaminants. The catalytic action of titanium oxide has to be activated by UV light, i.e. artificial illumination. However, modifying titanium oxide with neodymium(III) chloride allows catalysis under visible illumination, such as sun light. The modified catalyst is prepared by chemical coprecipitation–peptization method by ammonium hydroxide from mixture of TiCl4 and NdCl3 in aqueous solution). This process is used commercially on large scale on 1000 liter reactor for using in photocatalytic self-cleaning paints.[26][27]
Corrosion protection
Other applications are being developed. For example, it was reported that coating of aluminium or various aluminium alloys produces very corrosion-resistance surface, which then resisted immersion into concentrated aqueous solution of NaCl for two months without sign of pitting. The coating is produced either by immersion into aqueous solution of NdCl3 for a week or by electrolytic deposition using the same solution. In comparison with traditional chromium based corrosion inhibitors, NdCl3 and other rare-earth salts are environment friendly and much less toxic to humans and animals.[28][29]
The protective action of NdCl3 on aluminium alloys is based on formation of insoluble neodymium hydroxide. Being a chloride, NdCl3 itself is a corrosive agent, which is sometimes used for corrosion testing of ceramics.[30]
Labeling of organic molecules
Lanthanides, including neodymium are famous for their bright luminescence and therefore are widely used as fluorescent labels. In particular, NdCl3 has been incorporated into organic molecules, such as DNA, which could be then easily traced using a fluorescence microscope during various physical and chemical reactions.[21]
Health issues
Neodymium(III) chloride does not seem toxic to humans and animals (approximately similar to table salt). The LD50 (dose at which there is 50% mortality) for animals is about 3.7 g per kg of body weight (mouse, oral), 0.15 g/kg (rabbit, intravenous injection). Mild irritation of skin occurs upon exposure with 500 mg during 24 hrs (Draize test on rabbits).[31] Substances with LD50 above 2 g/kg are considered non-toxic.[32]
See also
- Lanthanoid
- Neodymium-doped yttrium lithium fluoride
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 4.75. ISBN 9781498754293.
- ↑ Morosin, B. (1968). "Crystal Structures of Anhydrous Rare‐Earth Chlorides". The Journal of Chemical Physics 49 (7): 3007–3012. doi:10.1063/1.1670543. Bibcode: 1968JChPh..49.3007M.
- ↑ O'Donoghue, Michael; Webster, Robert (2006). Gems. Butterworth-Heinemann. p. 523. ISBN 0-7506-5856-8. https://books.google.com/books?id=ZwcM5H-wHNoC&pg=PA523.
- ↑ 4.0 4.1 4.2 Edelmann, F. T.; Poremba, P. (1997). W. A. Herrmann. ed. Synthetic Methods of Organometallic and Inorganic Chemistry Vol. 6. Stuttgart: Georg Thieme Verlag.
- ↑ Steele, Marcus L.; Wertz, David L. (1977). "Solvent effects on the coordination of neodymium(3+) ions in concentrated neodymium trichloride solutions". Inorganic Chemistry 16 (5): 1225. doi:10.1021/ic50171a050.
- ↑ Skjeltorp, A (1977). "Analysis of magnetothermal parameters in NdCl3". Physica B+C 86-88: 1295–1297. doi:10.1016/0378-4363(77)90888-9. Bibcode: 1977PhyBC..86.1295S.
- ↑ Carlin, R. T. (1996). Molten Salts. The Electrochemical Society. p. 447. ISBN 1-56677-159-5. https://books.google.com/books?id=UWoEjb_BZFQC&pg=PA447.
- ↑ Meyer, Gerd; Morss, Lester R. (1991). Synthesis of lanthanide and actinide compounds. Springer. p. 161. ISBN 0-7923-1018-7. https://books.google.com/books?id=bnS5elHL2w8C&pg=PA161.
- ↑ 9.0 9.1 9.2 Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. pp. 444–446. ISBN 0-07-049439-8. https://books.google.com/books?id=Xqj-TTzkvTEC&pg=PA243. Retrieved 2009-06-06.
- ↑ Emsley, John (2003). Nature's building blocks: an A-Z guide to the elements. Oxford University Press. pp. 268–270. ISBN 0-19-850340-7. https://archive.org/details/naturesbuildingb0000emsl.
- ↑ 11.0 11.1 Nuyken, O.; Anwander, R. (2006). Neodymium based Ziegler catalysts. Springer. p. 15. ISBN 3-540-34809-3. https://books.google.com/books?id=xX9mYOp2HFYC&pg=PA15.
- ↑ Taylor, M. D.; Carter, P. C. (1962). "Preparation of anhydrous lanthanide halides, especially iodides". J. Inorg. Nucl. Chem. 24 (4): 387. doi:10.1016/0022-1902(62)80034-7.
- ↑ Kutscher, J.; Schneider, A. (1971). "Notiz zur Präparation von wasserfreien Lanthaniden-Haloge-niden, Insbesondere von Jodiden". Inorg. Nucl. Chem. Lett. 7 (9): 815. doi:10.1016/0020-1650(71)80253-2.
- ↑ Freeman, J. H.; Smith, M. L. (1958). "The preparation of anhydrous inorganic chlorides by dehydration with thionyl chloride". J. Inorg. Nucl. Chem. 7 (3): 224. doi:10.1016/0022-1902(58)80073-1.
- ↑ Druding, L. F.; Corbett, J. D. (1961). "Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide". J. Am. Chem. Soc. 83 (11): 2462. doi:10.1021/ja01472a010.
- ↑ Corbett, J. D. (1973). "Reduced Halides of the Rare Earth Elements.". Rev. Chim. Minérale 10: 239.
- ↑ Gupta, C. K.; Krishnamurthy, Nagaiyar (2004). Extractive metallurgy of rare earths. CRC Press. p. 276. ISBN 0-415-33340-7. https://books.google.com/books?id=E1npz8pwmYwC&pg=PA276.
- ↑ Henderson, B.; Bartram, Ralph H. (2000). Crystal field engineering of solid state laser materials. Cambridge University Press. p. 211. ISBN 0-521-59349-2. https://books.google.com/books?id=7wM9EQ1oDLwC&pg=PA211.
- ↑ Wolf, Emil (1993). Progress in Optics. Elsevier. p. 49. ISBN 0-444-81592-9. https://books.google.com/books?id=7mXhWv9f1tIC&pg=PA49.
- ↑ Wong, W; Liu, K; Chan, K; Pun, E (2006). "Polymer devices for photonic applications". Journal of Crystal Growth 288 (1): 100–104. doi:10.1016/j.jcrysgro.2005.12.017. Bibcode: 2006JCrGr.288..100W.
- ↑ 21.0 21.1 21.2 Comby, S; Bunzli, J (2007). "Chapter 235 Lanthanide Near-Infrared Luminescence in Molecular Probes and Devices". Handbook on the Physics and Chemistry of Rare Earths Volume 37. 37. pp. 217. doi:10.1016/S0168-1273(07)37035-9. ISBN 978-0-444-52144-6.
- ↑ Oriordan, A; Vandeun, R; Mairiaux, E; Moynihan, S; Fias, P; Nockemann, P; Binnemans, K; Redmond, G (2008). "Synthesis of a neodymium-quinolate complex for near-infrared electroluminescence applications". Thin Solid Films 516 (15): 5098. doi:10.1016/j.tsf.2007.11.112. Bibcode: 2008TSF...516.5098O. https://lirias.kuleuven.be/handle/123456789/182656.
- ↑ Cho, Y.; Choi, Y. K.; Sohn, S. H. (2006). "Optical properties of neodymium-containing polymethylmethacrylate films for the organic light emitting diode color filter". Applied Physics Letters 89 (5): 051102. doi:10.1063/1.2244042. Bibcode: 2006ApPhL..89e1102C.
- ↑ Marina, N; Monakov, Y; Sabirov, Z; Tolstikov, G (1991). "Lanthanide compounds—Catalysts of stereospecific polymerization of diene monomers. Review☆". Polymer Science U S S R 33 (3): 387. doi:10.1016/0032-3950(91)90237-K.
- ↑ Wang, C. (200). "In situ cyclization modification in polymerization of butadiene by rare earth coordination catalyst". Materials Chemistry and Physics 89: 116. doi:10.1016/j.matchemphys.2004.08.038.
- ↑ Xie, Y (2004). "Photocatalysis of neodymium ion modified TiO2 sol under visible light irradiation". Applied Surface Science 221 (1–4): 17–24. doi:10.1016/S0169-4332(03)00945-0. Bibcode: 2004ApSS..221...17X.
- ↑ Stengl, V; Bakardjieva, S; Murafa, N (2009). "Preparation and photocatalytic activity of rare earth doped TiO2 nanoparticles". Materials Chemistry and Physics 114: 217–226. doi:10.1016/j.matchemphys.2008.09.025.
- ↑ Agarwala, Vinod; Ugiansky, S. G. M. (1992). New methods for corrosion testing of aluminum alloys. ASTM International. p. 180. ISBN 0-8031-1435-4. https://books.google.com/books?id=0n3cWbIEPpgC&pg=PA180.
- ↑ Bethencourt, M; Botana, F.J.; Calvino, J.J.; Marcos, M.; Rodríguez-Chacón, M.A. (1998). "Lanthanide compounds as environmentally-friendly corrosion inhibitors of aluminium alloys: a review". Corrosion Science 40 (11): 1803. doi:10.1016/S0010-938X(98)00077-8.
- ↑ Takeuchi, M; Kato, T; Hanada, K; Koizumi, T; Aose, S (2005). "Corrosion resistance of ceramic materials in pyrochemical reprocessing condition by using molten salt for spent nuclear oxide fuel". Journal of Physics and Chemistry of Solids 66 (2–4): 521. doi:10.1016/j.jpcs.2004.06.046. Bibcode: 2005JPCS...66..521T.
- ↑ "Neodymium Chloride". American Elements. http://www.americanelements.com/ndcl.html.
- ↑ Garrett, Donald E. (1998). Borates. Academic Press. p. 385. ISBN 978-0-12-276060-0. https://books.google.com/books?id=imMJJP5T5rsC&pg=PA385.
 |