Chemistry:Nithiazine
From HandWiki
Short description: Chemical compound
| |
| Names | |
|---|---|
| IUPAC name
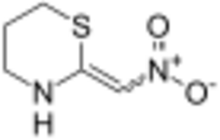
(E/Z)-2-Nitromethylene-1,3-thiazinane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H8N2O2S | |
| Molar mass | 160.19 g·mol−1 |
| Appearance | Crystals or brown powder |
| Density | 1.388 g/cm3 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P322, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nithiazine is a nitromethylene neonicotinoid insecticide. It is irritating to the eyes and skin, and is moderately toxic to mammals.[2]
Nithiazine does not act as an acetylcholinesterase inhibitor.[3]
References
- ↑ (Z)-nithiazine CSID:5013776, chemspider.com/Chemical-Structure (accessed 04:44, Jan 14, 2013)
- ↑ "nithiazine (Ref: BA 32476 )". University of Hertfordshire. http://sitem.herts.ac.uk/aeru/vsdb/Reports/2155.htm.
- ↑ Schroeder, M. E.; Flattum, R. F. (October 1984). "The Mode of Action and Neurotoxic Properties of the Nitromethylene Heterocycle Insecticides". Pesticide Biochemistry and Physiology 22 (2): 148–160. doi:10.1016/0048-3575(84)90084-1.
 |
