Chemistry:Fenitrothion
 | This article may be unbalanced towards certain viewpoints. (August 2018) |
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
O,O-Dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate | |
| Other names
• Dimethoxy-(3-methyl-4-nitrophenoxy)thioxophosphorane
O,O-Dimethyl O-4-nitro-m-tolyl phosphorothioate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H12NO5PS | |
| Molar mass | 277.23 g·mol−1 |
| Appearance | Yellow-brown liquid |
| Density | 1.3227 g/cm3 |
| Melting point | 3.4 °C (38.1 °F; 276.5 K) |
| Boiling point | 118 °C (244 °F; 391 K) at 0.05 mmHg |
| 38.0 mg/L | |
| Solubility | Readily soluble in dichloromethane, 2-propanol, toluene, hardly soluble in n-hexane.[1] |
| log P | 3.30 (octanol/water)[2] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
Rat, oral: 500 mg/kg[3] Mouse (female), oral: 1416 mg/kg[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
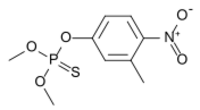
Fenitrothion (IUPAC name: O,O-dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate) is a phosphorothioate (organophosphate) insecticide that is inexpensive and widely used worldwide. Trade names include Sumithion, a 94.2% solution of fenitrothion.[5]
Health effects
Fenitrothion at sublethal doses affected the motor movement of marsupials,[6] and at acute dose levels it reduced the energy of birds.[7]
In chronic (low) dose tests, unexpectedly only the lowest concentration (0.011 microgram/liter) of fenitrothion depressed the growth of an algae, though all of the chronic dose levels used were toxic in other ways to the algae.[8]
Just half of fenitrothion's minimally effective dose altered the thyroid structure of a freshwater murrel (the snakehead fish).[9]
Cases of non-specific encephalopathy and fatty visceral changes (Reye's syndrome) in children living in the vicinity of fenitrothion-spraying operations invoked the research described latterly in Science,[10] and originally in The Lancet:[11]
| “ | 2-day-old mice were dosed topically for 11 days with fenitrothion, amongst other substances. After a further 2 days a sublethal dose of encephalomyocarditis virus was injected subcutaneously in known titre. Mortality-rates in the 10-day period after virus injection 4-9% in fenitrothion groups, and 0% in corn-oil controls. Fatty changes were noted in liver and kidney in the insecticide-virus groups. The encephalopathy showed no specific central-nervous system lesion, but death followed a sequence of paralysis and convulsions. The possible role of exposure to combinations of insecticides in human viral susceptibility requires further attention. | ” |
Further study showed that the illness was caused not by fenitrothion itself, but combinations which included the surfactants and the solvent (with or without the pesticide) clearly showed that pretreatment with these chemicals markedly increased the viral lethality in the test mice.[12]
Resistance
In an unusual demonstration of resistance to pesticides, 8% of insects in farm fields were found to carry a symbiotic gut microbe that can metabolize and detoxify fenitrothion; after in-vitro tests showed that the microbe significantly increased the survival of fenitrothion-treated insects.[13]
References
- ↑ Farm Chemicals Handbook. Willoughby, OH: Meister Publishing Co.. 1999. p. 177. ISBN 978-1-892829-02-3. OCLC 50201739.
- ↑ Exploring QSAR - Hydrophobic, Electronic, and Steric Constants.. Washington, DC: American Chemical Society. 1995. pp. 60. ISBN 978-0-8412-2993-8. OCLC 924843801.
- ↑ Kirk-Othmer Encyclopedia of Chemical Technology.. 3 (3rd ed.). New York, NY: John Wiley and Sons. 1981. p. 440. ISBN 978-0-471-02066-0. OCLC 873939596.
- ↑ Environ Health Criteria 133: Fenitrothion (Report). Geneva: World Health Organization. 1992. p. 70. http://www.inchem.org/documents/ehc/ehc/ehc133.htm.
- ↑ "Fenitrothion". PubChem Compound Database. National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/compound/fenitrothion.
- ↑ "Fenitrothion, an organophosphate, affects running endurance but not aerobic capacity in fat-tailed dunnarts (Sminthopsis crassicaudata)". Chemosphere 72 (9): 1315–20. July 2008. doi:10.1016/j.chemosphere.2008.04.054. PMID 18547601. Bibcode: 2008Chmsp..72.1315B.
- ↑ "Fipronil toxicity in northern bobwhite quail Colinus virginianus: reduced feeding behaviour and sulfone metabolite formation". Chemosphere 83 (4): 524–30. April 2011. doi:10.1016/j.chemosphere.2010.12.057. PMID 21227481. Bibcode: 2011Chmsp..83..524K.
- ↑ "Chronic toxicity of fenitrothion to an algae (Nannochloris oculata), a rotifer (Brachionus calyciflorus), and the cladoceran (Daphnia magna)". Ecotoxicology and Environmental Safety 35 (2): 112–20. November 1996. doi:10.1006/eesa.1996.0090. PMID 8950533.
- ↑ "Effect of safe concentrations of some pesticides on thyroid in the freshwater murrel, Channa punctatus: a histopathological study". Environmental Pollution 55 (2): 97–105. 1988. doi:10.1016/0269-7491(88)90121-2. PMID 15092506.
- ↑ "Lethal interaction of ubiquitous insecticide carriers with virus". Science 192 (4246): 1351–3. June 1976. doi:10.1126/science.179146. PMID 179146. Bibcode: 1976Sci...192.1351C.
- ↑ "Insecticide and viral interaction as a cause of fatty visceral changes and encephalopathy in the mouse". Lancet 2 (7871): 22–4. July 1974. doi:10.1016/S0140-6736(74)91351-8. PMID 4134409.
- ↑ "Analysis of an aromatic solvent used in a forest spray program". Chemosphere 6 (10): 641–651. 1977. doi:10.1016/0045-6535(77)90075-3. Bibcode: 1977Chmsp...6..641S.
- ↑ "Symbiont-mediated insecticide resistance". Proceedings of the National Academy of Sciences of the United States of America 109 (22): 8618–22. May 2012. doi:10.1073/pnas.1200231109. PMID 22529384. Bibcode: 2012PNAS..109.8618K.
Further reading
- Folster, David (14 May 1976). "Poison mists from pesticides in New Brunswick". Five Nights. CBC Radio. http://www.cbc.ca/archives/entry/canadas-trees-poison-mists.
External links
- Fenitrothion in the Pesticide Properties DataBase (PPDB)
- Hazardous Substances Data Bank (source of data)
- Inchem
- Entox
- Re-evaluation of Fenitrothion by the Pest Management Regulatory Agency of Canada
 |
