Chemistry:Afoxolaner
 | |
| Clinical data | |
|---|---|
| Pronunciation | /eɪˌfɒksoʊˈlænər/ ay-FOK-soh-LAN-ər |
| Trade names | Nexgard, Frontpro |
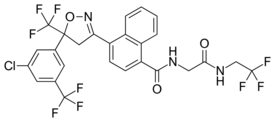
| Other names | 4-[(5RS)-5-(5-Chloro-α,α,α-trifluoro-m-tolyl)-4,5-dihydro-5-(trifluoromethyl)-1,2-oxazol-3-yl]-N-[2-oxo-2-(2,2,2-trifluoroethylamino)ethyl]naphthalene-1-carboxamide |
| License data |
|
| Routes of administration | By mouth |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 74% (Tmax = 2–4 hours)[1] |
| Elimination half-life | 14 hours[1] |
| Excretion | Bile duct (major route) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H17ClF9N3O3 |
| Molar mass | 625.88 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
Afoxolaner (INN[2]) is an insecticide and acaricide that belongs to the isoxazoline chemical compound group.
It acts as an antagonist at GABA-receptors (those gated by the neurotransmitter gamma-aminobutyric acid) and other ligand-gated chloride channels. Isoxazolines, among the chloride channel modulators, bind to a distinct and unique target site within the insect GABA-gated chloride channels, thereby blocking pre-and post-synaptic transfer of chloride ions across cell membranes. Prolonged afoxolaner-induced hyperexcitation results in uncontrolled activity of the central nervous system and death of insects and acarines.[3]
Safety
Toxicity for mammals
According to clinical studies performed prior to marketing:
- The oral toxicity profile of afoxolaner consists of a diuretic effect (rats only), effects secondary to a reduction in food consumption (rats and rabbits only) and occasional vomiting and/or diarrhoea (dogs, 120 and 200 mg/kg bodyweight (bw)) following high oral doses. No treatment-related effects on vomiting or diarrhoea were noted following oral doses of up to 31.5 mg/kg bw in the pivotal target animal safety study, nor in the EU field trial.[4]
- mild gastrointestinal effects (vomiting, diarrhoea), pruritus, lethargy, anorexia, and neurological signs (convulsions, ataxia and muscle tremors) have been reported in less than 0.1% of 10,000 animals treated, including isolated reports, most reported adverse reactions being self-limiting and of short duration,[5]
- (in combination with milbemycin oxime): vomiting, diarrhoea, lethargy, anorexia, and pruritus were observed in 0.2 to 1% of 10,000 animals treated and were generally self-limiting and of short duration,[3]
- In vitro studies reported that afoxolaner can bind to dopamine and norepinephrine cellular transport receptor systems and the CB1 receptor; inhibition of these catecholaminergic systems and certain types of competitive binding at CB1 receptors may mediate pharmacodynamic effects of diuresis, decreased food consumption, and decreased body weight in animals.[4]
According to post-marketing safety experience:
- (in combination with milbemycin oxime): erythema and neurological signs (convulsions, ataxia and muscle tremors) have been reported in less than 0.1% of 10,000 animals treated, including isolated reports,[3]
- The US Food and Drug Administration FDA reports[6] that some drugs in this class (isoxazolines), including afoxolaner, can have adverse neurologic effects on some dogs, such as muscle tremors, ataxia, and seizures.
- Extralabel use of afoxolaner in a pet pig has been described without any adverse effects.[7] Experimental use in commercial pigs also did not result in any adverse effects.[8]
Selectivity in insects over mammalians
In vivo studies (repeat-dose toxicology in laboratory animals, target animal safety, field studies) provided by MERIAL, the company that produces afoxolaner-derivative medicines, did not show evidence of neurological or behavioural effects suggestive of GABA-mediated perturbations in mammals. The Committee for Medicinal Products for Veterinary Use (CVMP) therefore concluded that binding to dog, rat or human GABA receptors is expected to be low for afoxolaner.[4]
Selectivity for insect over mammalian GABA-receptors has been demonstrated for other isoxazolines.[9] The selectivity might be explained by the number of pharmacological differences that exist between GABA-gated chloride channels of insects and vertebrates.[10]
Legal status
The marketing authorization was granted by the European Medicines Agency in February 2014, for Nexgard and January 2015, for Nexgard Spectra, after only 14[11] and 12[4] months of quality, safety and efficacy assessment performed by the Committee for Medicinal Products for Veterinary Use (CVMP).[12] Therefore, long-term effects are not known.
Brand names
Afoxolaner is the active ingredient of the veterinary medicinal products Nexgard, Frontpro, and Nexgard Spectra (in combination with milbemycin oxime).[13][14][15] They are indicated for the treatment and prevention of flea infestations, and the treatment and control of tick infestations in dogs and puppies (8 weeks of age and older, weighing 4 pounds (~1.8 kilograms) of body weight or greater) for one month.[16] These products are administered orally and poisons fleas once they start feeding.
References
- ↑ 1.0 1.1 1.2 "Frontline NexGard (afoxolaner) for the Treatment and Prophylaxis of Ectoparasitic Diseases in Dogs. Full Prescribing Information" (in ru). Sanofi Russia. http://www.merial.ru/SiteCollectionDocuments/instructions/Frontline_Nexgard_06_10_2015.pdf.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 70". World Health Organization. pp. 276–7. https://www.who.int/medicines/publications/druginformation/innlists/RL70.pdf.
- ↑ 3.0 3.1 3.2 "NexGard Spectra product information - Annex I "Summary of product characteristics"". https://www.ema.europa.eu/en/documents/product-information/nexgard-spectra-epar-product-information_en.pdf.
- ↑ 4.0 4.1 4.2 4.3 "CVMP assessment report for NexGard (EMEA/V/C/002729/0000)". https://www.ema.europa.eu/en/documents/assessment-report/nexgard-epar-public-assessment-report_en.pdf.
- ↑ "NexGard product information - Annex I "Summary of product characteristics"". https://www.ema.europa.eu/en/documents/product-information/nexgard-epar-product-information_en.pdf.
- ↑ "CVM Updates - Animal Drug Safety Communication: FDA Alerts Pet Owners and Veterinarians About Potential for Neurologic Adverse Events Associated with Certain Flea and Tick Products". Center for Veterinary Medicine. U.S. Food and Drug Administration. https://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm620934.htm.
- ↑ "Afoxolaner as a Treatment for a Novel Sarcoptes scabiei Infestation in a Juvenile Potbelly Pig". Frontiers in Veterinary Science 7: 473. 2020. doi:10.3389/fvets.2020.00473. PMID 33102538.
- ↑ "Efficacy and Pharmacokinetics Evaluation of a Single Oral Dose of Afoxolaner against Sarcoptes scabiei in the Porcine Scabies Model for Human Infestation". Antimicrobial Agents and Chemotherapy 62 (9). September 2018. doi:10.1128/AAC.02334-17. PMID 29914951.
- ↑ "Golden age of RyR and GABA-R diamide and isoxazoline insecticides: common genesis, serendipity, surprises, selectivity, and safety". Chemical Research in Toxicology 28 (4): 560–566. April 2015. doi:10.1021/tx500520w. PMID 25688713.
- ↑ "Molecular biology of insect neuronal GABA receptors". Trends in Neurosciences 20 (12): 578–583. December 1997. doi:10.1016/S0166-2236(97)01127-2. PMID 9416671.
- ↑ "CVMP Assessment Report for NEXGARD SPECTRA(EMEA/V/C/003842/0000)". https://www.ema.europa.eu/en/documents/assessment-report/nexgard-spectra-epar-public-assessment-report_en.pdf.
- ↑ "Committee for Medicinal Products for Veterinary Use (CVMP) - Section "Role of the CVMP"". 17 September 2018. https://www.ema.europa.eu/en/committees/committee-medicinal-products-veterinary-use-cvmp.
- ↑ "Discovery and mode of action of afoxolaner, a new isoxazoline parasiticide for dogs". Veterinary Parasitology 201 (3–4): 179–189. April 2014. doi:10.1016/j.vetpar.2014.02.020. PMID 24631502.
- ↑ "Afoxolaner against fleas: immediate efficacy and resultant mortality after short exposure on dogs". Parasite 21: 42. 25 August 2014. doi:10.1051/parasite/2014045. PMID 25148564.
- ↑ "Evaluation of the efficacy of monthly oral administration of afoxolaner plus milbemycin oxime (NexGard Spectra(®), Merial) in the prevention of adult Spirocerca lupi establishment in experimentally infected dogs". Veterinary Parasitology 226: 150–161. August 2016. doi:10.1016/j.vetpar.2016.07.002. PMID 27514901.
- ↑ "Boehringer-Ingelheim companion-animals-product NexGard (afoxolaner).". Boehringer Ingelheim International GmbH. https://www.boehringer-ingelheim.com/animal-health/companion-animals-products/nexgard?itid=NexGard%C2%AE.
 |
