Chemistry:Lotilaner
 | |
| Clinical data | |
|---|---|
| Trade names | Credelio, Xdemvy |
| Other names | TP-03 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth, eye drops |
| Drug class | Antiparasitic |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
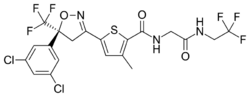
| Formula | C20H14Cl3F6N3O3S |
| Molar mass | 596.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lotilaner, sold under the brand name Xdemvy, is an ectoparasiticide (anti-parasitic) medication used for the treatment of blepharitis (inflammation of the eyelid) caused by infestation by Demodex (tiny mites).[1][5] It is used as an eye drop.[1]
It was approved for medical use in the United States in July 2023.[1][5][6][7] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[8]
Medical uses
Lotilaner is indicated for the treatment of Demodex blepharitis.[1]
Veterinary uses
Lotilaner, sold under the brand name Credelio among others, is a veterinary medication used to control fleas and ticks in dogs and cats.[2][3][4][9] It is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and for the treatment and control of tick infestations including lone star tick (Amblyomma americanum), American dog tick (Dermacentor variabilis), black-legged tick (Ixodes scapularis), and brown dog tick (Rhipicephalus sanguineus).[10] It is taken by mouth.[2][3]
Lotilaner in combination with milbemycin oxime is sold under the brand name Credelio Plus.[11] It is used in dogs to treat concurrent infestations with parasites living outside (ticks and/or fleas) and inside (worms) the animal's body.[11]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Xdemvy- lotilaner ophthalmic solution solution/ drops". 26 July 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ccd9e37c-654e-4e84-8c85-6523457df979.
- ↑ 2.0 2.1 2.2 "Credelio- lotilaner tablet, chewable". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=427f2ebc-ce24-452b-bbb3-43d4ef8b63b0.
- ↑ 3.0 3.1 3.2 "Credelio- lotilaner tablet, chewable". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=365b701f-e9a8-458f-90ff-33ca1fb947ca.
- ↑ 4.0 4.1 "Credelio EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/veterinary/EPAR/credelio.
- ↑ 5.0 5.1 "Novel Drug Approvals for 2023". 25 July 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023.
- ↑ "Xdemvy: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=217603.
- ↑ "FDA Approves Xdemvy (lotilaner ophthalmic solution) 0.25% for the treatment of Demodex blepharitis" (Press release). Tarsus Pharmaceuticals. 25 July 2023. Retrieved 5 August 2023 – via GlobeNewswire.
- ↑ (PDF) New Drug Therapy Approvals 2023 (Report). January 2024. https://www.fda.gov/media/175253/download. Retrieved 9 January 2024.
- ↑ "Safety evaluation of lotilaner in dogs after oral administration as flavoured chewable tablets (Credelio)". Parasites & Vectors 10 (1): 538. November 2017. doi:10.1186/s13071-017-2468-y. PMID 29089043.
- ↑ "Freedom Of Information Summary, Supplemental New Animal Drug Application, NADA 141-494, Credelio, Lotilaner, Chewable Tablets, Dogs". 3 September 2019. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/7663.
- ↑ 11.0 11.1 "Credelio Plus EPAR". 19 February 2021. https://www.ema.europa.eu/en/medicines/veterinary/EPAR/credelio-plus. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
 |
