Chemistry:Fluralaner
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌflʊərəˈlænər/ FLOOR-ə-LAN-ər |
| Trade names | Bravecto, Exzolt |
| Other names |
|
| License data |
|
| Routes of administration | By mouth |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20–27%;[1] reduced in the fasted state[2] |
| Elimination half-life | 9.3–16.2 days[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
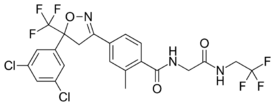
| Formula | C22H17Cl2F6N3O3 |
| Molar mass | 556.29 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
Fluralaner (INN)[4] is a systemic insecticide and acaricide that is administered orally[5] or topically.[6] The U.S. Food and Drug Administration (FDA) approved it under the trade name Bravecto for flea treatment in dogs in May 2014[7] and Bravecto Plus as a topical treatment for cats in November 2019,[8] with warnings about possible side effects in both species.[9] The EU approved the drug in February 2014.[10] Australia approved it for the treatment and prevention of ticks and fleas on dogs in January 2015.[11] For treating mites in chickens, a solution for use in drinking water is available under the name Exzolt;[12] it was introduced by the EU in 2017.[13]
Mode of action
Fluralaner inhibits γ-aminobutyric acid (GABA)-gated chloride channels (GABAA receptors) and L-glutamate-gated chloride channels (GluCls).[14] Potency of fluralaner is comparable to fipronil (a related GABA-antagonist insecticide and acaricide).[15]
Research
Fluralaner is being investigated to determine its ability to reduce the incidence of mosquito-borne diseases,[16] as well as bed bugs.[17][18]
References
- ↑ 1.0 1.1 "Bravecto (fluralaner) for the Treatment and Prophylaxis of Arachnoenthomoses in Dogs. Full Prescribing Information" (in Russian). Intervet GesmbH. http://www.msd-animal-health.ru/Binaries/bravecto0316_tcm53-179097.pdf.
- ↑ "The effect of food on the pharmacokinetics of oral fluralaner in dogs". Parasites & Vectors 7 (1): 84. March 2014. doi:10.1186/1756-3305-7-84. PMID 24598049.
- ↑ "Bravecto (fluralaner) Flavored Chews for Dogs. Prescribing Information". Intervet, Inc., a subsidiary of Merck & Company, In.. http://us.bravovets.com/pdfs/bravecto_pi_mah.pdf.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 69". WHO Drug Information 27 (1): 59. 2013. http://apps.who.int/medicinedocs/documents/s20154en/s20154en.pdf. Retrieved 14 November 2016.
- ↑ "Safety of fluralaner chewable tablets (Bravecto), a novel systemic antiparasitic drug, in dogs after oral administration". Parasites & Vectors 7 (1): 87. March 2014. doi:10.1186/1756-3305-7-87. PMID 24606886.
- ↑ "A single topical fluralaner application to cats and to dogs controls fleas for 12 weeks in a simulated home environment". Parasites & Vectors 11 (1): 385. July 2018. doi:10.1186/s13071-018-2927-0. PMID 29970135.
- ↑ "New Flea/Tick Medication by Merck Just Approved: Bravecto". 21 May 2014. http://drjustinelee.com/new-fleatick-medication-bravecto-just-approved/.
- ↑ "BRAVECTO® PLUS (fluralaner and moxidectin topical solution) for Cats Receives Approval from US Food and Drug Administration" (in english). 2019-11-15. https://www.merck-animal-health.com/blog/2019/11/15/bravecto-plus-fluralaner-and-moxidectin-topical-solution-for-cats-receives-approval-from-us-food-and-drug-administration/.
- ↑ Center for Veterinary Medicine (2020-07-31). "Fact Sheet for Pet Owners and Veterinarians about Potential Adverse Events Associated with Isoxazoline Flea and Tick Products" (in en). https://www.fda.gov/animal-veterinary/animal-health-literacy/fact-sheet-pet-owners-and-veterinarians-about-potential-adverse-events-associated-isoxazoline-flea.
- ↑ "MSD Animal Health receives EU approval for Bravecto". 19 February 2014. https://www.zenopa.com/news/801695271/msd-animal-health-receives-eu-approval-for-bravecto.
- ↑ "Agricultural and Veterinary Chemicals". Australian Pesticides and Veterinary Medicines Authority. 10 February 2015. https://apvma.gov.au/sites/default/files/gazette_10022015.pdf.
- ↑ Imrie, Paul (2020-06-24). "Backyard poultry red mite treatment launches". https://www.vettimes.co.uk/news/backyard-poultry-red-mite-treatment-launches/.
- ↑ Brauneis, Maria D.; Zoller, Hartmut; Williams, Heike; Zschiesche, Eva; Heckeroth, Anja R. (2017). "The acaricidal speed of kill of orally administered fluralaner against poultry red mites (Dermanyssus gallinae) on laying hens and its impact on mite reproduction". Parasites & Vectors 10 (1): 594. doi:10.1186/s13071-017-2534-5. PMID 29197422.
- ↑ "The novel isoxazoline ectoparasiticide fluralaner: selective inhibition of arthropod γ-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity". Insect Biochemistry and Molecular Biology 45: 111–124. February 2014. doi:10.1016/j.ibmb.2013.11.009. PMID 24365472.
- ↑ "Differential mechanisms of action of the novel γ-aminobutyric acid receptor antagonist ectoparasiticides fluralaner (A1443) and fipronil". Pest Management Science 71 (1): 91–95. January 2015. doi:10.1002/ps.3768. PMID 24591229.
- ↑ "Repurposing isoxazoline veterinary drugs for control of vector-borne human diseases". Proceedings of the National Academy of Sciences of the United States of America 115 (29): E6920–E6926. July 2018. doi:10.1073/pnas.1801338115. PMID 29967151. Bibcode: 2018PNAS..115E6920M.
- ↑ Murez, Cara (1 December 2022). "Two veterinary drugs may help eliminate bedbugs". United Press International, Inc.. https://www.upi.com/Health_News/2022/12/01/veterinary-medicine-bedbugs/6091669903318/.
- ↑ Sheele, Johnathan M. (2020). "A Preliminary Report Showing Spinosad and Fluralaner Are Able to Incapacitate Cimex lectularius L., the Common Bed Bug". Cureus 12 (4): e7529. doi:10.7759/cureus.7529. PMID 32377477.
 |
