Chemistry:Rhodium(III) iodide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| I3Rh | |
| Molar mass | 483.61890 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H413 | |
| P273, P501 | |
| Related compounds | |
Other anions
|
Rhodium(III) bromide; Rhodium(III) chloride; Rhodium(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhodium(III) iodide is an inorganic compound with the formula RhI3. It is a black solid.[1]
Preparation
Rhodium(III) iodide can be synthesised by the reaction of aqueous potassium iodide with rhodium(III) bromide.[1]
- RhBr3 + 3KI → RhI3 + 3KBr
Structure
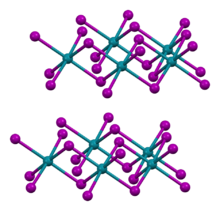
RhI3 adopts same crystal structure motif as AlCl3 and YCl3. The structure consists of cubic close-packed iodide ions and rhodium ions filling a third of the octahedral interstices, forming a layers.[2]
Reactivity
Rhodium(III) iodide is only known in the anhydrous form. Unlike the other rhodium(III) halides, it does not form hydrates.[1] The related anion [RhI6]3− was previously thought not to form[1] but has since been prepared by diffusion of RhCl3·3H2O through a layer of hydroiodic acid into piperazine.[3]
References
- ↑ 1.0 1.1 1.2 1.3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1119-1120. ISBN 978-0-08-037941-8.
- ↑ Brodersen, K.; Thiele, G.; Recke, I. (1968). "Strukturuntersuchungen an Rhodiumhalogeniden". J. Less-Common Met. 14 (1): 151–152. doi:10.1016/0022-5088(68)90214-2.
- ↑ Bujak, Maciej (2015). "Efficient Diffusion-Controlled Ligand Exchange Crystal Growth of Isostructural Inorganic–Organic Halogenidorhodates(III): The Missing Hexaiodidorhodate(III) Anion". Cryst. Growth Des. 15 (3): 1295–1302. doi:10.1021/cg501694d.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

