Chemistry:Zaprinast
| |
| Names | |
|---|---|
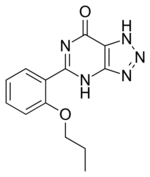
| IUPAC name
5-(2-Propoxyphenyl)-1H-[1,2,3]triazolo[4,5-d]pyrimidin-7(4H)-one
| |
| Other names
M&B 22,948
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H13N5O2 | |
| Molar mass | 271.280 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zaprinast was an unsuccessful clinical drug candidate that was a precursor to the chemically related PDE5 inhibitors, such as sildenafil (Viagra), which successfully reached the market. It is a phosphodiesterase inhibitor,[1] selective for the subtypes PDE5, PDE6, PDE9 and PDE11. IC50 values are 0.76, 0.15, 29.0, and 12.0 μM, respectively.[2][3]
Zaprinast inhibits the growth of asexual blood-stage malaria parasites (P. falciparum) in vitro with an ED50 value of 35 μM, and inhibits PfPDE1, a P. falciparum cGMP-specific phosphodiesterase, with an IC50 value of 3.8 μM.[4]
Zaprinast has also been shown to activate the orphan G-protein coupled receptor known as GPR35, both in rats and humans,[5] and to inhibit the mitochondrial pyruvate carrier. [6]
References
- ↑ Choi, SH; Choi, DH; Song, KS; Shin, KH; Chun, BG (2002). "Zaprinast, an inhibitor of cGMP-selective phosphodiesterases, enhances the secretion of TNF-alpha and IL-1beta and the expression of iNOS and MHC class II molecules in rat microglial cells". Journal of Neuroscience Research 67 (3): 411–21. doi:10.1002/jnr.10102. PMID 11813247.
- ↑ Taniguchi, Y.; Tonaikachi, H.; Shinjo, K. (2006). "Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35". FEBS Letters 580 (21): 5003–5008. doi:10.1016/j.febslet.2006.08.015. PMID 16934253.
- ↑ Keswani, A. N.; Peyton, K. J.; Durante, W.; Schafer, A. I.; Tulis, D. A. (2009). "The Cyclic GMP Modulators YC-1 and Zaprinast Reduce Vessel Remodeling Through Antiproliferative and Proapoptotic Effects". Journal of Cardiovascular Pharmacology and Therapeutics 14 (2): 116–124. doi:10.1177/1074248409333266. PMID 19342499.
- ↑ Keizo Yuasa; Fumika Mi-Ichi; Tamaki Kobayashi; Masaya Yamanouchi; Jun Kotera; Kiyoshi Kita; Kenji Omori (2005). "PfPDE1, a novel cGMP-specific phosphodiesterase from the human malaria parasite Plasmodium falciparum". Biochem. J. 392 (Pt 1): 221–9. doi:10.1042/BJ20050425. PMID 16038615.
- ↑ Yasuhito Taniguchi; Hiroko Tonai-Kachi; Katsuhiro Shinjo (2006). "Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35". FEBS Letters 580 (21): 5003–5008. doi:10.1016/j.febslet.2006.08.015. PMID 16934253.
- ↑ Jianhai Du; Whitney M Cleghorn; Laura Contreras; Ken Lindsay; Austin M Rountree; Andrei O Chertov; Sally J Turner; Ayse Sahaboglu et al. (2013). "Inhibition of mitochondrial pyruvate transport by zaprinast causes massive accumulation of aspartate at the expense of glutamate in the retina". J Biol Chem 288 (50): 36129–36140. doi:10.1074/jbc.M113.507285. PMID 24187136.
 |
