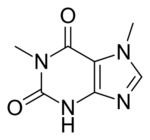
Chemistry:Paraxanthine
| |
| |
| Names | |
|---|---|
| IUPAC name
1,7-Dimethyl-3H-purine-2,6-dione
| |
| Other names
Paraxanthine,
1,7-Dimethylxanthine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H8N4O2 | |
| Molar mass | 180.167 g·mol−1 |
| Melting point | 351 to 352 °C (664 to 666 °F; 624 to 625 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Paraxanthine, also known as 1,7-dimethylxanthine, is a metabolite of theophylline and theobromine, two well-known stimulants found in coffee, tea, and chocolate. It is a member of the xanthine family of alkaloids, which includes theophylline, theobromine and caffeine.
Production and metabolism
Paraxanthine is not known to be produced by plants[1] but is observed in nature as a metabolite of caffeine in animals and some species of bacteria.[2]
Paraxanthine is the primary metabolite of caffeine in humans and other animals, such as mice.[3] Shortly after ingestion, roughly 84% of caffeine is metabolized into paraxanthine by hepatic cytochrome P450, which removes a methyl group from the N3 position of caffeine.[4][5][6] After formation, paraxanthine can be broken down to 7-methylxanthine by demethylation of the N1 position,[7] which is subsequently demethylated into xanthine or oxidized by CYP2A6 and CYP1A2 into 1,7-dimethyluric acid.[6] In another pathway, paraxanthine is broken down into 5-acetylamino-6-formylamino-3-methyluracil through N-acetyl-transferase 2, which is then broken down into 5-acetylamino-6-amino-3-methyluracil by non-enzymatic decomposition.[8] In yet another pathway, paraxanthine is metabolized CYPIA2 forming 1-methyl-xanthine, which can then be metabolized by xanthine oxidase to form 1-methyl-uric acid.[8]
Certain proposed synthetic pathways of caffeine make use of paraxanthine as a bypass intermediate. However, its absence in plant alkaloid assays implies that these are infrequently, if ever, directly produced by plants.[citation needed]
Pharmacology and physiological effects
Like caffeine, paraxanthine is a psychoactive central nervous system (CNS) stimulant.[2]
Pharmacodynamics
Studies indicate that, similar to caffeine, simultaneous antagonism of adenosine receptors[9] is responsible for paraxanthine's stimulatory effects. Paraxanthine adenosine receptor binding affinity (21 μM for A1, 32 μM for A2A, 4.5 μM for A2B, and >100 for μM for A3) is similar or slightly stronger than caffeine, but weaker than theophylline.[10]
Paraxanthine is a selective inhibitor of cGMP-preferring phosphodiesterase (PDE9) activity[11] and is hypothesized to increase glutamate and dopamine release by potentiating nitric oxide signaling.[12] Activation of a nitric oxide-cGMP pathway may be responsible for some of the behavioral effects of paraxanthine that differ from those associated with caffeine.[13]
Paraxanthine is a competitive nonselective phosphodiesterase inhibitor[14] which raises intracellular cAMP, activates PKA, inhibits TNF-alpha[15][16] and leukotriene[17] synthesis, and reduces inflammation and innate immunity.[17]
Unlike caffeine, paraxanthine acts as an enzymatic effector of Na+/K+ ATPase. As a result, it is responsible for increased transport of potassium ions into skeletal muscle tissue.[18] Similarly, the compound also stimulates increases in calcium ion concentration in muscle.[19]
Pharmacokinetics
The pharmacokinetic parameter for paraxanthine are similar to those for caffeine, but differ significantly from those for theobromine and theophylline, the other major caffeine-derived methylxanthine metabolites in humans (Table 1).
| Plasma Half-Life
(t1/2; hr) |
Volume of Distribution
(Vss,unbound; l/kg) |
Plasma Clearance
(CL; ml/min/kg) | |
|---|---|---|---|
| Caffeine | 4.1 ± 1.3 | 1.06 ± 0.26 | 2.07 ± 0.96 |
| Paraxanthine | 3.1 ± 0.8 | 1.18 ± 0.37 | 2.20 ± 0.91 |
| Theobromine | 7.2 ± 1.6 | 0.79 ± 0.15 | 1.20 ± 0.40 |
| Theophylline | 6.2 ± 1.4 | 0.77 ± 0.17 | 0.93 ± 0.22 |
Uses
Paraxanthine is a phosphodiesterase type 9 (PDE9) inhibitor and it is sold as a research molecule for this same purpose.[21]
Toxicity
Paraxanthine is believed to exhibit a lower toxicity than caffeine and the caffeine metabolite, theophylline.[22][23] In a mouse model, intraperitoneal paraxanthine doses of 175 mg/kg/day did not result in animal death or overt signs of stress;[24] by comparison, the intraperitoneal LD50 for caffeine in mice is reported at 168 mg/kg.[25] In in vitro cell culture studies, paraxanthine is reported to be less harmful than caffeine and the least harmful of the caffeine-derived metabolites in terms of hepatocyte toxicity.[26]
As with other methylxanthines, paraxanthine is reported to be teratogenic when administered in high doses;[24] but it is a less potent teratogen as compared to caffeine and theophylline. A mouse study on the potentiating effects of methylxanthines coadministered with mitomycin C on teratogenicity reported the incidence of birth defects for caffeine, theophylline, and paraxanthine to be 94.2%, 80.0%, and 16.9%, respectively; additionally, average birth weight decreased significantly in mice exposed to caffeine or theophylline when coadministered with mitomycin C, but not for paraxanthine coadministered with mitomycin C.[27]
Paraxanthine was reported to be significantly less clastogenic compared to caffeine or theophylline in an in vitro study using human lymphocytes.[28]
References
- ↑ Stavric, B. (1988-01-01). "Methylxanthines: Toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines" (in en). Food and Chemical Toxicology 26 (8): 725–733. doi:10.1016/0278-6915(88)90073-7. ISSN 0278-6915. PMID 3058562.
- ↑ 2.0 2.1 "Catabolism of caffeine in plants and microorganisms". Frontiers in Bioscience 9 (1–3): 1348–59. May 2004. doi:10.2741/1339. PMID 14977550.
- ↑ "Biotransformation of methylxanthines in mammalian cell lines genetically engineered for expression of single cytochrome P450 isoforms. Allocation of metabolic pathways to isoforms and inhibitory effects of quinolones". Toxicology 82 (1–3): 169–89. October 1993. doi:10.1016/0300-483x(93)90064-y. PMID 8236273.
- ↑ "Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels". Molecular Pharmacology 74 (4): 980–9. October 2008. doi:10.1124/mol.108.048207. PMID 18621927.
- ↑ "Caffeine and exercise: metabolism and performance". Canadian Journal of Applied Physiology 19 (2): 111–38. June 1994. doi:10.1139/h94-010. PMID 8081318.
- ↑ 6.0 6.1 "Catabolism of caffeine in plants and microorganisms". Frontiers in Bioscience 9 (1–3): 1348–59. May 2004. doi:10.2741/1339. PMID 14977550.
- ↑ "Genetic characterization of caffeine degradation by bacteria and its potential applications". Microbial Biotechnology 8 (3): 369–78. May 2015. doi:10.1111/1751-7915.12262. PMID 25678373.
- ↑ 8.0 8.1 Caffeine : chemistry, analysis, function and effects. Preedy, Victor R.,, Royal Society of Chemistry (Great Britain). Cambridge, U.K.. 2012. ISBN 9781849734752. OCLC 810337257.
- ↑ "Adenosine receptors: development of selective agonists and antagonists". Progress in Clinical and Biological Research 230 (1): 41–63. 1987. PMID 3588607.
- ↑ Müller, Christa E.; Jacobson, Kenneth A. (2011), Fredholm, Bertil B., ed., "Xanthines as Adenosine Receptor Antagonists" (in en), Methylxanthines, Handbook of Experimental Pharmacology (Springer) 200 (200): pp. 151–199, doi:10.1007/978-3-642-13443-2_6, ISBN 978-3-642-13443-2, PMID 20859796
- ↑ Orrú, Marco; Guitart, Xavier; Karcz-Kubicha, Marzena; Solinas, Marcello; Justinova, Zuzana; Barodia, Sandeep Kumar; Zanoveli, Janaina; Cortes, Antoni et al. (April 2013). "Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans". Neuropharmacology 67C: 476–484. doi:10.1016/j.neuropharm.2012.11.029. ISSN 0028-3908. PMID 23261866.
- ↑ Ferré, Sergi; Orrú, Marco; Guitart, Xavier (2013). "Paraxanthine: Connecting Caffeine to Nitric Oxide Neurotransmission". Journal of Caffeine Research 3 (2): 72–78. doi:10.1089/jcr.2013.0006. ISSN 2156-5783. PMID 24761277.
- ↑ Orrú, Marco (2013). "Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans". Neuropharmacology 67C: 476–484. doi:10.1016/j.neuropharm.2012.11.029. PMID 23261866.
- ↑ "Cyclic nucleotide phosphodiesterases". The Journal of Allergy and Clinical Immunology 108 (5): 671–80. November 2001. doi:10.1067/mai.2001.119555. PMID 11692087.
- ↑ "Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition". Clinics 63 (3): 321–8. June 2008. doi:10.1590/S1807-59322008000300006. PMID 18568240.
- ↑ "Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages". American Journal of Respiratory and Critical Care Medicine 159 (2): 508–11. February 1999. doi:10.1164/ajrccm.159.2.9804085. PMID 9927365.
- ↑ 17.0 17.1 "Leukotrienes: underappreciated mediators of innate immune responses". Journal of Immunology 174 (2): 589–94. January 2005. doi:10.4049/jimmunol.174.2.589. PMID 15634873. http://www.jimmunol.org/cgi/content/full/174/2/589.
- ↑ "K+ transport in resting rat hind-limb skeletal muscle in response to paraxanthine, a caffeine metabolite". Canadian Journal of Physiology and Pharmacology 77 (11): 835–43. November 1999. doi:10.1139/y99-095. PMID 10593655.
- ↑ "Paraxanthine, a caffeine metabolite, dose dependently increases [Ca(2+)](i) in skeletal muscle". Journal of Applied Physiology 89 (6): 2312–7. December 2000. doi:10.1152/jappl.2000.89.6.2312. PMID 11090584.
- ↑ Lelo, A.; Birkett, D. J.; Robson, R. A.; Miners, J. O. (August 1986). "Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man". British Journal of Clinical Pharmacology 22 (2): 177–182. doi:10.1111/j.1365-2125.1986.tb05246.x. ISSN 0306-5251. PMID 3756065.
- ↑ "Paraxanthine". https://www.caymanchem.com/pdfs/21068.pdf.
- ↑ Neal L. Benowitz; Peyton Jacob; Haim Mayan; Charles Denaro (1995). "Sympathomimetic effects of paraxanthine and caffeine in humans". Clinical Pharmacology & Therapeutics 58 (6): 684–691. doi:10.1016/0009-9236(95)90025-X. PMID 8529334. http://www.nature.com/clpt/journal/v58/n6/abs/clpt1995184a.html.
- ↑ Institute of Medicine (US) Committee on Military Nutrition Research (2001). Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations. Washington (DC): National Academies Press (US). ISBN 978-0-309-08258-7. http://www.ncbi.nlm.nih.gov/books/NBK223802/.
- ↑ 24.0 24.1 York, R. G.; Randall, J. L.; Scott, W. J. (1986). "Teratogenicity of paraxanthine (1,7-dimethylxanthine) in C57BL/6J mice". Teratology 34 (3): 279–282. doi:10.1002/tera.1420340307. ISSN 0040-3709. PMID 3798364.
- ↑ (in en) Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health. 1987. https://books.google.com/books?id=fDVaIb9H7DAC&q=caffeine+mouse+LD50+intraperitoneal+168+mg%2Fkg&pg=PA1373.
- ↑ Gressner, Olav A.; Lahme, Birgit; Siluschek, Monika; Gressner, Axel M. (2009). "Identification of paraxanthine as the most potent caffeine-derived inhibitor of connective tissue growth factor expression in liver parenchymal cells". Liver International 29 (6): 886–897. doi:10.1111/j.1478-3231.2009.01987.x. ISSN 1478-3231. PMID 19291178.
- ↑ Nakatsuka, Toshio; Hanada, Satoshi; Fujii, Takaaki (1983). "Potentiating effects of methylxanthines on teratogenicity of mitomycin C in mice" (in en). Teratology 28 (2): 243–247. doi:10.1002/tera.1420280214. ISSN 1096-9926. PMID 6417813.
- ↑ Weinstein, David; Mauer, Irving; Katz, Marion L.; Kazmer, Sonja (1975). "The effect of methylxanthines on chromosomes of human lymphocytes in culture" (in en). Mutation Research/Environmental Mutagenesis and Related Subjects 31 (1): 57–61. doi:10.1016/0165-1161(75)90064-3. ISSN 0165-1161. PMID 1128545.
External links
 |
