Chemistry:BRL-50481
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H12N2O4S |
| Molar mass | 244.27 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
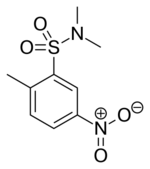
BRL-50481 is a drug developed by GlaxoSmithKline which is the first compound that acts as a phosphodiesterase inhibitor selective for the PDE7 family.[1] PDE7 activity is encoded by two genes, PDE7A and PDE7B. BRL-50481 actually shows about an 80-fold preference for the PDE7A subtype, for which it was developed, over PDE7B.[2] BRL-50481 has been shown to increase mineralisation activity in osteoblasts, suggesting a potential role for PDE7 inhibitors in the treatment of osteoporosis.[3]
References
- ↑ "Discovery of BRL 50481 [3-(N,N-dimethylsulfonamido)-4-methyl-nitrobenzene, a selective inhibitor of phosphodiesterase 7: in vitro studies in human monocytes, lung macrophages, and CD8+ T-lymphocytes"]. Molecular Pharmacology 66 (6): 1679–89. December 2004. doi:10.1124/mol.104.002246. PMID 15371556. http://molpharm.aspetjournals.org/content/molpharm/66/6/1679.full.pdf.
- ↑ "New classes of PDE7 inhibitors identified by a fission yeast-based HTS". Journal of Biomolecular Screening 15 (4): 359–67. April 2010. doi:10.1177/1087057110362100. PMID 20228279.
- ↑ "Effects of phosphodiesterase 7 inhibition by RNA interference on the gene expression and differentiation of human mesenchymal stem cell-derived osteoblasts". Bone 43 (1): 84–91. July 2008. doi:10.1016/j.bone.2008.02.021. PMID 18420479.
 |
