Biology:Crisaborole
 | |
| Clinical data | |
|---|---|
| Pronunciation | /juːˈkrɪsə/ yoo-KRIS-ə |
| Trade names | Eucrisa, Staquis |
| Other names | AN-2728 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617019 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical (ointment) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
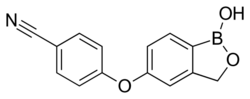
| Formula | C14H10BNO3 |
| Molar mass | 251.05 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Crisaborole, sold under the brand name Eucrisa among others, is a nonsteroidal topical medication used for the treatment of mild-to-moderate atopic dermatitis (eczema) in adults and children.[1][2][3][4]
The most common side effects are reactions at the application site (including burning or stinging).[3]
Crisaborole is a phosphodiesterase 4 (PDE-4) inhibitor, although its specific mechanism of action in atopic dermatitis is not known.[1][2]
Side effects
At the site of application, crisaborole may cause burning or stinging. Rarely, there may be an allergic reaction.[5]
Medical uses
In the US, crisaborole is indicated for topical treatment of mild to moderate atopic dermatitis in people three months of age and older.[2]
In the EU, crisaborole was authorized for treatment of mild to moderate atopic dermatitis in people two years of age and older with ≤ 40% body surface area (BSA) affected.[3]
Pharmacology
Pharmacodynamics
Crisaborole is a phosphodiesterase-4 inhibitor, mainly acting on phosphodiesterase 4B (PDE4B), which causes inflammation.[6] Chemically, crisaborole is a phenoxybenzoxaborole.[6] Inhibition of PDE4B appears to suppress the release of tumor necrosis factor alpha (TNFα), interleukin-12 (IL-12), IL-23 and other cytokines, proteins believed to be involved in the immune response and inflammation.[6]
People with atopic dermatitis produce high levels of proteins called cytokines, which can cause the inflammation of the skin seen in dermatitis.[3] Crisaborole blocks the release of certain cytokines involved in the inflammation process such as tumor necrosis factor alpha, interleukins (IL‑2, IL-4, IL-5), and interferon gamma.[3] By blocking their release, crisaborole is expected to ease the inflammation and therefore relieve symptoms of the disease.[3]
Chemistry
Crisaborole (chemical name: 4-[(1-hydroxy-1,3-dihydro-2,1-benzoxaborol-5-yl)oxy]benzonitrile) is a member of the class of benzoxaboroles characterized by the presence of a boronic acid hemiester with a phenolic ether and a nitrile.[7] Crisaborole crystallizes into two polymorphs that differ in the conformation of the oxaborole ring. A cocrystal with 4,4'-bipyridine has been prepared and studied by X-ray crystallography.[8]
History
Crisaborole was developed by Anacor Pharmaceuticals for the topical treatment of psoriasis.[9][6][10] During preclinical and clinical development, crisaborole was called AN2728 and PF-06930164.[11] The drug was assumed to be potential $2bn-a-year blockbuster, when Pfizer acquired Anacor Pharmaceuticals.[12] However, the drug was commercially not successful, reaching only US$147 million in sales in 2018, and US$138 million in sales in 2019.[13]
Crisaborole was approved for use in the United States in December 2016[14][1] and for use in Canada in June 2018.[15]
The safety and efficacy of crisaborole were established in two placebo-controlled trials with a total of 1,522 participants ranging in age from two years of age to 79 years of age, with mild to moderate atopic dermatitis.[1] In both trials participants received treatment with either crisaborole or placebo twice daily for 28 days.[16] Neither the participants nor the health care providers knew which treatment was being given until after the trials were completed.[16] Overall, participants receiving crisaborole achieved greater response with clear or almost clear skin after 28 days of treatment.[1][16] The trials were conducted in the US.[16]
Crisaborole, approved for the treatment of mild to moderate atopic dermatitis in the European Union, has been rapidly withdrawn from the European market (March 2020 - February 2022).[3]
See also
- Tavaborole – a structurally related topical antifungal developed by Anacor
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "FDA Approves Eucrisa for Eczema" (Press release). U.S. Food and Drug Administration (FDA). 14 December 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 2.0 2.1 2.2 "Eucrisa- crisaborole ointment". 21 April 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=609b77de-1ca3-4783-b8f5-01a9c0f1d77d.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Staquis EPAR". 29 January 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/staquis. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Topical Administration of Crisaborole in Mild to Moderate Atopic Dermatitis: A Systematic Review and Meta-Analysis" (in en). Dermatologic Therapy 2023: 1–9. 2023-02-06. doi:10.1155/2023/1869934. ISSN 1529-8019.
- ↑ "PRODUCT MONOGRAPH". 2018-06-07. https://pdf.hres.ca/dpd_pm/00045835.PDF.
- ↑ 6.0 6.1 6.2 6.3 "A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology". Dermatology Online Journal 20 (5): 22608. May 2014. doi:10.5070/D3205022608. PMID 24852768. http://escholarship.org/uc/item/2hx1m6kv.pdf.
- ↑ "WHO Drug Information, Vol. 29, No. 3, 2015. International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 74". World Health Information. p. 391. https://www.who.int/medicines/publications/druginformation/innlists/RL74.pdf.
- ↑ "Exploration of Solid Forms of Crisaborole: Crystal Engineering Identifies Polymorphism in Commercial Sources and Facilitates Cocrystal Formation". Crystal Growth & Design 18 (8): 4416–4419. 2018. doi:10.1021/acs.cgd.8b00375. ISSN 1528-7483.
- ↑ "AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis". Current Opinion in Investigational Drugs 10 (11): 1236–42. November 2009. PMID 19876791.
- ↑ "Neue Wirkstoffe: Crisaborol" (in German). Österreichische Apotheker-Zeitung (17/2016). 16 August 2016.
- ↑ "Crisaborole". AdisInsight. http://adisinsight.springer.com/drugs/800024239.
- ↑ "Pfizer to Acquire Anacor". Pfizer (Press release). 16 May 2016. Retrieved 28 April 2020.
- ↑ "Pfizer Financial Report". Pfizer. https://s21.q4cdn.com/317678438/files/doc_financials/Quarterly/2019/q4/Q4-2019-PFE-Earnings-Release.pdf.
- ↑ "Eucrisa (crisaborole) Ointment". 23 January 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/207695Orig1s000TOC.cfm.
- ↑ "Eucrisa Regulatory Decision Summary". 2014-10-23. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00420.
- ↑ 16.0 16.1 16.2 16.3 "Drug Trials Snapshot: Eucrisa". 14 December 2016. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshot-eucrisa.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
- "Crisaborole". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/crisaborole.
 |
